Abstract
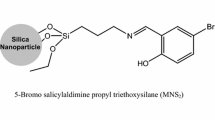
Modification of SiO2 nanoparticles by salicylaldiminepropyl results in efficient adsorbents for removal of Th4+, UO 2+2 and Eu3+ ions from aqueous solutions. The effect of parameters influencing the adsorption efficiency such as aqueous phase pH, contact time, initial metal ions concentration, adsorbent dosage and temperature dependency of the process was verified and discussed. Under optimal conditions (pH 5.5, adsorbent dosage 0.05 g, contact time 30 min. and 25 °C), thorium and uranyl ions (initial concentration 20 mg/l) were quantitatively removed from 20 ml of sample solution. Under such conditions 85% of europium ions was removed. Comparison of the adsorption efficiency of the studied modified nano-particles with those unmodified ones shows a shift for uptake of the metal ions vs. pH curves towards lower pH values by applying the modified adsorbents. In addition, a significant improvement of europium ions adsorption was observed by using the modified nanoparticles. Kinetics of the process was studied by considering a pseudo second-order model. This model predicts chemisorption for the adsorption mechanism. Freundlich, Langmuir and Temkin models were suitable for describing the equilibrium data of Th4+, UO2 2+ and Eu3+ adsorption process, respectively. Thermodynamic investigation reveals the adsorption process of the studied ions is entropy driven.
Similar content being viewed by others
References
J. F. Malone, D. J. Marrs, M. A. McKervey, P. O’Hagan, N. Thompson, A. Walker, F. Aranaud-Neu, O. Mauprivez, M.-J. Schwing-Weill, J.-F. Dozol, H. Rouquette and N. Simon, J. Chem. Soc., dChem. Commun., 2151 (1995).
F. Arnaud-Neu, V. Böhmer, J. F. Dozol, C. Grüttner, R.A. Jakobi, D. Kraft, O. Mauprivez, H. Rouquette, M.-J. Schwing-Weill, N. Simon and W. Vogt, J. Chem. Soc., Perkin Trans. II, 1175 (1996).
K. L. Nash, Solvent Extr. Ion Exch., 11, 729 (1993).
C. Madic, J. Bourgesm and J.-F. Dozol, International conference on accelerators-driven transmutation technology and applications, Las Vegas (1994).
C. S. K. Raju, M. S. Subramanian, N. Sivaraman, T.G. Srinivasan and B. R. V. Roab, J. Chromatogr. A, 1156, 340 (2007).
V. K. Jain, R. A. Pandya, S.G. Pillai and P. S. Shrivastav, Talanta, 70, 257 (2006).
J.K. Joona, J. S. Mikko, J.H.H. Hanna and K.T.T. Simo, Opt. Exp., 14, 11539 (2006).
B. Gupta, P. Malik and A. Deep, J. Radioanal. Nucl. Chem., 251, 451 (2002).
M. Eskandari Nasab, A. Samm and S. A. Milani, Hydrometallurgy, 106, 141 (2011).
S. K. Sahu, V. Chakravortty, M. L. P. Reddy and T.R. Ramamohan, Talanta, 51, 523 (2000).
X. Zhong, Y. Wu and J. Radioanal. Nucl. Chem., 292, 355 (2012).
P. Kumar, A. Pal, M. K. Saxena and K. L. Ramakumar, Desalination, 232, 71 (2008).
A. Fujiwara, Y. Kameo, A. Hoshi, T. Haraga and M. Nakashima, J. Chromatogr. A, 1140, 163 (2007).
P.G. Jaison, V. M. Telmore, P. Kumar and S. K. Aggarwal, J. Chromatogr. A., 1216, 1383 (2009).
D. Hritcu, D. Humelnicu, G. Dodi and M. I. Popa, Carbohyd. Polym., 87, 1155 (2012).
M. D. Pereira and M. A. Z. Arruda, Microchim. Acta, 141, 115 (2003).
A. R. Ghiasvand, R. Ghaderi and A. Kakanejadifard, Talanta, 62, 287 (2004).
P. K. Jal, S. Patel and B. K. Mishra, Talanta, 62, 1005 (2004).
V. Gurnani, A. K. Singh and B. Venkataramani, Anal. Chim. Acta, 485, 221 (2003).
M. F. El-shahat, E. A. Moawed and M. A.A. Zaid, Talanta, 59, 851 (2003).
A. Uzun, M. Soylak and I. L. Elc, Talanta, 54, 197 (2001).
S.M. Nelms, G.M. Greenway and D. Koller, J. Anal. At. Spectrom., 11, 907 (1996).
S. Patil, A. Sandberg, E. Heckert, W. Self and S. Seal, Biomolecules, 28, 4600 (2007).
M. Janschm P. Stumf, C. Graf, E. Rühi and R. H. Müller, Int. J. Pharm., 428, 125 (2012).
A. Saxena, H. Mangal, P. K. Rai, A. S. Rawat, V. Kumar and M. Datta, J. Hazard. Matter., 180, 566 (2010).
S. Ghosh, A. Z.M. Badruddoza, M. S. Uddin and K. Hidajat, J. Colloid Interface Sci., 354, 483 (2011).
Y. Feng, J.-L. Gong, G.-M. Zeng, Q.-Y. Niu, J.-H. Deng and M. Yan, Chem. Eng. J., 162, 487 (2010).
Y.C. Sharma, V. Srivastava, V.K. Singh, S.N. Kauf and C. H. Weng, Environ. Technol., 30, 583 (2009).
A. Nilchi, T. Shariati Dehaghan and S, Rasoul Garmarodi, Desalination, DOI:10.16/j.desal.2012.06.022.
S. Sadeghi, H. Azhdari, H. Arabi and A. Zeraatkar Moghadam, J. Hazard. Matter., 215–216, 208 (2012).
S. Sayin and M. Yilmaz, Desalination, 276, 328 (2011).
See for example: (a) Z. Shiri-Yekta, M. R. Yaftian and A. Nilchi, J. Iran. Chem. Soc., 10, 221 (2013)
N. Sehati, Z. Shiri-Yekta, A. A. Zamani, M. R. Yaftian and N. Noshiranzadeh, Sep. Sci. Technol., 47, 670 (2012)
M. R. Yaftian, M. R. Razipour and D. Matt, J. Radioanal. Nucl. Chem., 270, 357 (2006)
M. R. Yaftian, R. Taheri, A. A. Zamani and D. Matt, J. Radioanal. Nucl. Chem., 262, 255 (2004)
A. A. Zamani and M. R. Yaftian, Sep. Purif. Technol., 40, 115 (2004)
M. R. Yaftian, M. E. Eshraghi and L. Hassanzadeh, Iran. J. Chem. Chem. Eng., 22, 71 (2003)
M. R. Yaftian, L. Hassanzadeh, M. E. Eshraghi and D. Matt, Sep. Purif. Technol., 31, 261 (2003).
See for example: (a) S. A.M. Fathi, Sh. Rostamkhani and M. R. Yaftian, J. Anal. Chem., 65, 614 (2010)
M. Parinejad and M. R. Yaftian, Iran. J. Chem. Chem. Eng., 28, 85 (2009)
S. A.M. Fathi and M. R. Yaftian, J. Colloid Interface Sci., 334, 167 (2009)
S. A.M. Fathi and M. R. Yaftian, J. Hazard. Mater., 164, 133 (2009).
M. Ghorbanloo, H. H. Monfared and C. Janiak, J. Mol. Catal. A: Chem., 345, 12 (2011).
J. A. Dean, Analytical chemistry handbook, McGraw-Hill, New York (1995).
Y. S. Ho and A. E. Ofomaja, J. Hazard. Mater., B129, 137 (2006).
H. Jiang, Y. Xu, J. Zhang, L. Zhang and R. Han, Life Sci. J., 4, 42 (2007).
V. Vadivelan and K. V. Kumar, J. Colloid Interface Sci., 286, 90 (2005).
M.R. Mehrasbi, Z. Farahmandkia, B. Taghibeigloo and A. Taromi, Water Air Soil Pollut., 199, 343 (2009).
M. Nameni, M. R. Alavi Moghadam and M. Arami, Int. J. Environ. Sci. Technol., 5, 161 (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shiri-Yekta, Z., Yaftian, M.R. & Nilchi, A. Silica nanoparticles modified with a Schiff base ligand: An efficient adsorbent for Th(IV), U(VI) and Eu(III) ions. Korean J. Chem. Eng. 30, 1644–1651 (2013). https://doi.org/10.1007/s11814-013-0077-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-013-0077-9