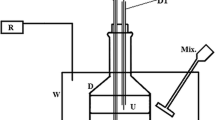
Abstract
Experimental liquid-liquid equilibrium (LLE) data for the system of (water+butyric acid+iso-butyl acetate) were obtained at T=(298.2, 303.2, 308.2, and 313.2) K and atmospheric pressure. This ternary system exhibits type-1 behavior of LLE. The experimental tie-line data were correlated using the UNIQUAC and NRTL models. The reliability of the experimental tie-line data was determined by applying the Othmer-Tobias and Hand correlations. Distribution coefficients and separation factors were calculated over the immiscibility regions.
Similar content being viewed by others
References
A. Senol, Fluid Phase Equilib., 243, 51 (2006).
D. Özmen, J. Chem. Thermodyn., 39, 123 (2007).
D. Özmen, Fluid Phase Equilib., 269, 12 (2008).
H. Uslu and Ş. I. Kırbaşlar, Fluid Phase Equilib., 287, 134 (2010).
S. Şahin, Ş. I. Kırbaşlar and M. Bilgin, J. Chem. Thermodyn., 41, 97 (2009).
Ş. I. Kırbaşlar, S. Şahinn and M. Bilgin, J. Chem. Thermodyn., 39, 1463 (2007).
M. Bilgin and C. Arisoy, Fluid Phase Equilib., 250, 59 (2006).
S. A. Al-Muhtaseb and M. A. Fahim, Fluid Phase Equilib., 123, 189 (1996).
D. Vandak, J. Zigova, E. Sÿturdik and S. Schlosser, Process Biochem., 32, 245 (1977).
E. Sabolova, S. Schlosser and J. Martak, J. Chem. Eng. Data, 46, 735 (2001).
Z.Y. Li, W. Qin and Y. Dai, J. Chem. Eng. Data, 47, 843 (2002).
Ş. I. Kırbaşlar, S. Şahin and M. Bilgin, J. Chem. Thermodyn., 39, 1279 (2007).
Ş. I. Kırbaşlar, J. Chem. Thermodyn., 38, 696 (2006).
Ş. I. Kırbaşlar, M. Bilgin and D. Batr, J. Chem. Thermodyn., 37, 175 (2005).
M. Bilgin, J. Chem. Thermodyn., 38, 1643 (2006).
M. Bilgin, Ş. I. Kırbaşlar, Ö. Özcan and U. Dramur, J. Chem. Thermodyn., 37, 297 (2005).
A. Gök, Ş. I. Kırbaşlar, H. Uslu and H. Ghanadzadeh Gilani, Fluid Phase Equilib., 303, 71 (2011).
H. Ghanadzadeh, A. Ghanadzadeh, S. Asgharzadeh and N. Dastmoozeh, Thermochim. Acta, 523, 154 (2011).
H. Ghanadzadeh, A. Ghanadzadeh, Z. Aghajani, S. Abbasnejad and S. Shekarsaraee, J. Chem. Thermodyn., 42, 695 (2010).
D. S. Abrams and J.M. Prausnitz. AIChE J., 21, 116 (1975).
H. Renon and J.M. Prausnitz. AIChE J., 14, 135 (1968).
N. Peschke and S. I. Sandler, J. Chem. Eng. Data, 40, 315 (1995).
H. Li and K. Tamura, Fluid Phase Equilib., 263, 223 (2008).
A. Merzougui, A, Hasseine, A, Kabouche and M. Korichi, Fluid Phase Equilib., 309, 161 (2011).
A. Bondi, J. Phys. Chem., 68, 441 (1964).
A. Bondi, Physical Properties of Molecular Crystals, Liquids and Glasses, John Wiley and Sons Inc., New York (1968).
T. Banerjee, M. K. Singh, R.K. Sahoo and A. Khanna, Fluid Phase Equilib., 234, 64 (2005).
D. F. Othmer and P. E. Tobias, Ind. Eng. Chem., 34, 690 (1942).
V. Brandani, A. Chianese and M. Rossi, J. Chem. Eng. Data, 30, 27 (1985).
A. Chafer, J. de la Torre, J. B. Monton and E. Lladosa, Fluid Phase Equilib., 265, 122 (2008).
H. Ghanadzadeh Gilani, M. Golpour and B. Abbasi Souraki, J. Chem. Thermodyn., 49, 39 (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghanadzadeh, H., Asgharzadeh, S., Dastmoozeh, N. et al. Tie-line data for aqueous mixtures of butyric acid with isobutyl acetate at various temperatures. Korean J. Chem. Eng. 29, 1615–1621 (2012). https://doi.org/10.1007/s11814-012-0067-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-012-0067-3