Abstract
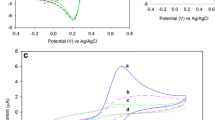
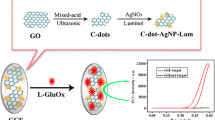
Electrogenerated Chemiluminescence (ECL) involves applying a certain electric potential to a chemical reaction, resulting in the oxidation or reduction of the substance which reacts to produce light. We determined the amount of glucose by its reaction to glucose oxidase (GO X ) on the surface of the proposed modified electrode, which results hydrogen peroxide (H2O2) as side product. After that the reactions between luminol and H2O2 under oxidizing conditions generate dependent light which can be used to analyze. In the current article at first we proposed a convenient method to obtaining a self-assembly modified electrode. A nano based modified glassy carbon (GC) electrode (Glucose oxidase/Ag nanoparticles/cysteamine (CA)/p-aminobenzene sulfonic acid/GC electrode) was prepared, and the ECL behavior of luminol in the presence of glucose was examined. Compared to the bare GC electrode, the modified electrode incorporating glucose oxidase significantly enhanced the response of the ECL biosensor to glucose due to the enhanced specificity of the modified surface to enzymatic reaction, and the sensitivity of the luminol ECL reaction. Under optimal conditions, the electrode was established to respond linearly to glucose in the concentration range 5.0×10−7 to 8.0×10−3 mol/L, and the detection limit was established to be a glucose concentration of 4.0×10−8 mol/L.
Similar content being viewed by others
References
B. Qiu, Z. Lin, J. Wang, Z. Chen, J. Chen and G. Chen, Talanta, 78, 76 (2009).
L. Hu and G. Xu, Chem. Soc. Rev., 39, 3275 (2010).
S.M. Borisov and O. S. Wolfbeis, Chem. Rev., 108, 423 (2008).
X. M. Chen, B.Y. Su, X. H. Song, Q. A. Chen, X. Chen and X. R. Wang, TrAC, Trends Anal. Chem., 30, 665 (2011).
L. Zhang, D. Li, W. Meng, Q. Huang, Y. Su, L. Wang, S. Song and C. Fan, Biosens. Bioelectron., 25, 368 (2009).
S. Xu, Y. Liu, T. Wang and J. Li, Anal. Chem., 82, 9566 (2010).
H. Qi, Y. Peng, Q. Gao and C. Zhang, Biosensors, 9, 674 (2009).
X. Chen, Z. Cai, Z. Lin, T. Jia, H. Liu, Y. Jiang and X. Chen, Biosens. Bioelectron., 24, 3475 (2009).
X. Yang, R. Yuan, Y. Chai, Y. Zhuo, L. Mao and Y. Shirong, Biosensors and Bioelectronics, 25,7, 1851 (2010).
P. Bertoncello and R. J. Forster, Biosens. Bioelectron., 24, 3191 (2009).
K. Lin and S. Chen, J. Electroanal. Chem., 589, 52 (2006).
X. Liu, W. Niu, H. Li, S. Han, L. Hu and G. Xu, Electrochem. Commun., 10, 1250 (2008).
W. Wang, H. Cui, Z.X. Deng, Y. P. Dong and J. Z. Guo, J. Electroanal. Chem., 612, 277 (2008).
Z. Lin, J. Sun, J. Chen, L. Guo, Y. Chen and G. Chen, Anal. Chem., 80, 2826 (2008).
C. A. Marquette, B. D. Leca and L. J. Blum, Luminescence., 16, 159 (2001).
B. Leca and L. J. Blum, The Anal., 125, 789 (2000).
H. Dai, X. Wu, H. Xu, Y. Wang, Y. Chi and G. Chen, Electrochim. Acta, 54(19), 4582 (2009).
Z. Lin, J. Chen and G. Chen, Electrochim. Acta, 53(5), 2396 (2008).
Z. Guo, Y. Xue and X. Zheng, J. Electroanal. Chem., 625(1), 47 (2009).
M.Q. Liu, J. H. Jiang, Y. L. Feng, G. L. Shen and R. Q. Yu, Chinese J. Anal. Chem., 35(10), 1435 (2007).
B.W. Park, D. S. Kim and D.Y. Yoon, Korean J. Chem. Eng., 28(1), 64 (2011).
B. Haghighi and S. Bozorgzadeh, Anal. Chim. Acta, 697(1–2), 90 (2011).
H. H. Chu, W.Y. Guo, J.W. Di, Y. Wu and Y. F. Tu, Electroanalysis, 21, 1630 (2009).
J. S. Huang, D.W. Wang, H.Q. Hou and T.Y. You, Advanced Functional Materials, 18, 441 (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rad, A.S., Ardjmand, M., Jahanshahi, M. et al. Self-assembly electrode based on silver nanoparticle toward electrogenerated chemiluminescence analysis of glucose. Korean J. Chem. Eng. 29, 1063–1068 (2012). https://doi.org/10.1007/s11814-011-0280-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-011-0280-5