Abstract
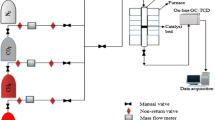
The adsorption of methane on two activated carbons with different physical properties was measured. Adsorption isotherms were obtained by static volumetric method at different temperatures and pressures. The experimental results sow the best gas storage capacity was 113.5 V/V at temperature 280 K and pressure 8.5MPa on an activated carbon with surface area 1,060 m2/gr. An artificial neural network (ANN) based on genetic algorithm (GA) was used to predict amount of adsorption. The experimental data including input pressure, temperature and surface area of adsorbents as input parameters were used to create a GA-ANN simulation. The simulation results were compared with the experimental data and a good agreement was observed. The simulation was applied to calculate isosteric heat of adsorption by using the Clausius-Clapeyron equation. Comparison of the calculated adsorption heat showed different surface heterogeneity of the adsorbents.
Similar content being viewed by others
References
M. Tagliabuea, D. Farrussengb, S. Valenciac, S. Aguadob, U. Ravonb, C. Rizzoa, A. Cormac and C. Mirodatosa, J. Chem. Eng., 155, 553 (2009).
D. Lozano-Castelló, J. Alcañiz-Monge, M. A. de la Casa-Lillo, D. Cazorla-Amorós and A. Linares-Solano, J. Fuel, 81, 1777 (2002).
A. Danna, S. Iyuke, A. FakhrulRazi, T. Chuah, M. A. Atieh and M. F. Aikhatib, Environ. J. Info. Sci., 1, 597 (2003).
J. Wegrzyn and M. Gurevih, J. Appl. Energy, 55, 71 (1996).
Y. Zhou, Y. Wang, H. Chen and L. Zhou, J. Carbon, 43, 2007 (2005).
A. Linares-Solano Adsorbed natural gas storage for alternative motor fuels, Conference of the 6th framework program, Warsaw, Poland (2002).
L. L. Vasiliev, L. E. Kanonchik, D. A. Mishkinis and M. I. Rabetsky, Int. J. Therm. Sci., 39, 1047 (2000).
S. Cavenati, C. A. Grande and A. E. Rodrigues, J. Chem. Eng. Data, 49, 1095 (2004).
H. Najibi, A. Chapoy and B. Tohidi, J. Fuel, 87, 7 (2007).
Li Zhou and Yan Sun, AIChE J., 48, 2412 (2002).
S.H. Himeno, T. Komatsu and S. H. Fujita, J. Chem. Eng. Data, 50, 369 (2005).
R. Ch. Bansal and M. Goyal, Activated Carbon Adsorption, CRC Press (2005).
M. Rasoolzadeh and Sh. Fatemi, Iran. J. Chem. Chem. Eng., 27,3, 127 (2008).
S. Aber, N. Daneshvar, S. M. Soroureddin, A. Chabok and K. Asadpour- Zeynali, Desalination, 211, 87 (2007).
J. Bryjak, K. Ciesielski and I. Zbiciski, J. Biotechnol., 114, 177 (2004).
K. Vasanth Kumar, M. Monteiro de Castro, M. Martinez-Escandell, M. Molina-Sabio and F. Rodriguez-Reinoso, Chem. Eng. J., 159, 272 (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Molashahi, M., Hashemipour, H. Experimental study and artificial neural network simulation of methane adsorption on activated carbon. Korean J. Chem. Eng. 29, 601–605 (2012). https://doi.org/10.1007/s11814-011-0215-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-011-0215-1