Abstract
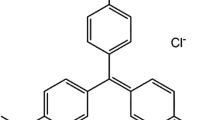
Adsorption characteristics of methylene blue (MB) from aqueous solution on natural poplar leaf were investigated. Batch experiments were carried out to study the effects of initial pH, contact time, adsorbent dosage, and initial MB concentration, salt concentration (Ca2+ and Na+) as well as temperature on MB adsorption. The optimum condition for adsorption was found at pH 6–9 and adsorbent dosage of 2 g L−1. The equilibration time was 240 min. The salt concentration had a negative effect on MB removal. The equilibrium data were analyzed with Langmuir, Freundlich and Koble-Corrigan isotherm models using nonlinear regression method. The adsorption process was more effectively described by Langmuir isotherm based on the values of the correlation coefficient R2 and chi-square statistic x2. The maximum monolayer adsorption capacity of poplar leaf from the Langmuir model was 135.35 mg g−1 at 293 K. The pseudo second order equation provided a better fit to experimental data in the kinetic studies. Intraparticle diffusion was involved in adsorption process, but it was not the only rate-controlling step. Thermodynamic quantities such as ΔG, ΔH and ΔS were calculated, indicating that the adsorption process was spontaneous and endothermic. Dye-adsorbent interactions were examined by FTIR and SEM analysis. The FTIR results suggested that there were hydroxyl and carboxyl groups on the surface of poplar leaf, which would make MB adsorption possible. The SEM images showed effective adsorption of MB molecules on the adsorbent surface.
Similar content being viewed by others
References
T. Robinson, B. Chandran and P. Nigam, Water Res., 36, 2824 (2002).
Ö. Gerçel, H. F. Gerçel A, Savaş Koparal and Ülker Bakır Öǧütveren, J. Hazard. Mater., 160, 668 (2008).
T. Robinson, B. Chandran and P. Nigam, Bioresour. Technol., 85, 119 (2002).
K.V. Kumar and K. Porkodi, J. Hazard. Mater., 146, 214 (2007).
O. Gulnaz, A. Kaya and S. Dincer, J. Hazard. Mater., B134, 190 (2006).
S. D. Khattri and M. K. Singh, J. Hazard. Mater., 167, 1089 (2009).
A. E. Ofomaja and Y. S. Ho, Dyes Pigm., 74, 60 (2007).
V. Ponnusami, V. Krithika, R. Madhuram and S. N. Srivastava, J. Hazard. Mater., 142, 397 (2007).
T. G. Chuah, A. Jumasiah, I. Azni, S. Katayon and S. Y. Thomas Choong, Desalination, 175, 305 (2005).
Y. S. Ho, T. H. Chiang and Y.M. Hsueh, Process Biochem., 40, 119 (2005).
K. V. Kumar, Dyes Pigm., 74, 595 (2007).
C. H. Weng, Y. T. Lin and T.W. Tzeng, J. Hazard. Mater., 170, 417 (2009).
M. Dundar, C. Nuhoglu and Y. Nuhoglu, J. Hazard. Mater., 151, 86 (2008).
R. Salim, M. Al-Subu, I. Abu-Shqair and H. Braik, Inst. Chem. Eng., 81B, 236 (2003).
G. Bayramöglu and M.Y. Arıca, J. Hazard. Mater., 143, 135 (2007).
T. Akar, I. Tosun, Z. Kaynak, E. Ozkara, O. Yeni, E. N. Sahin and S. T. Akar, J. Hazard. Mater., 166, 1217 (2009).
P. Waranusantigul, P. Pokethitiyook, M. Kruatrachue and E. S. Upatham, Environ. Pollut., 125, 385 (2003).
V. Vadivelan and K. V. Kumar, J. Colloid Interface Sci., 286, 90 (2005).
S. Wang, Z. H. Zhu, A. Coomes, F. Haghseresht and G. Q. Lu, J. Colloid Interface Sci., 284, 440 (2005).
E. Lorenc-Grabowska and G. Gryglewicz, Dyes Pigm., 74, 34 (2007).
R. P. Han, W. H. Zou, W. H. Yu, S. J. Cheng, Y. F. Wang and J. Shi, J. Hazard. Mater., 141, 156 (2007).
R. P. Han, Y. Wang, P. Han, J. Shi, J. Yang and Y. S. Lu, J. Hazard. Mater., B137, 550 (2006).
Y. Bulut and H. Aydin, Desalination, 194, 259 (2006).
K. V. Kumar and A. Kumaran, Biochem. Eng. J., 27, 83 (2005).
Y. Wang, Y. Mu, Q. B. Zhao and H. Q. Yu, Sep. Purif. Technol., 50, 1 (2006).
J.Y. Song, W. H. Zou, Y.Y. Bian, F.Y. Su and R. P. Han, Desalination, 265, 119 (2011).
B. Royer, N. F. Cardoso, E. C. Lima, Julio C. P. Vaghetti, N. M. Simon, T. Calvete and R. Cataluna Veses, J. Hazard. Mater., 164, 1213 (2009).
X. L. Han, W. Wang and X. J. Ma, Chem. Eng. J., 171, 1 (2011).
Z. Aksu, Process Biochem., 38, 89 (2002).
G. Annadurai, R.-S. Juang and D.-J. Lee, J. Hazard. Mater., B92, 263 (2002).
Z. Aksu and G. Karabayır, Bioresour. Technol., 99, 7730 (2008).
Q. L. Fu, Y. L. Deng, H. S. Li, J. Liu, H. Q. Hu, S.W. Chen and T. M. Sa, Appl. Surf. Sci., 255, 4551 (2009).
M. Ugurlu, Micropor. Mesopor. Mater., 119, 276 (2009).
T. Akar, A. S. Ozcan, S. Tunali and A. Ozcan, Bioresour. Technol., 99, 3057 (2008).
M. Dögan, M. Alkan, A. Türkyilmaz and Y. Özdemir, J. Hazard. Mater., B109, 141 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, X., Niu, X. & Ma, X. Adsorption characteristics of methylene blue on poplar leaf in batch mode: Equilibrium, kinetics and thermodynamics. Korean J. Chem. Eng. 29, 494–502 (2012). https://doi.org/10.1007/s11814-011-0211-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-011-0211-5