Abstract
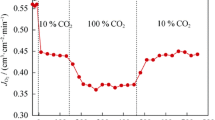
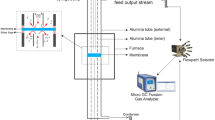
The effect of minor surface modification on the performance of Ba0.5Sr0.5Co0.8Fe0.2O3−δ membrane was evaluated in the temperature region from 700 to 850 °C. Oxygen permeation experiments were conducted according to membrane thickness (1.0mm and 1.6 mm) and oxygen partial pressure (0.21, 0.42, and 0.63 atm) in the absence and in the presence of carbon dioxide (300 and 500 ppm). The oxygen permeation flux of Ba0.5Sr0.5Co0.8Fe0.2O3−δ membrane increased with increasing temperature and decreasing membrane thickness. The oxygen permeation flux through the membrane of 1.0 mm thickness with Ba0.5Sr0.5Co0.8Fe0.2O3−δ -modified surface was ca. 1.23 ml/cm2·min at 850 °C under air feeding condition. It was found that the Ba0.5Sr0.5Co0.8Fe0.2O3−δ -modified Ba0.5Sr0.5Co0.8Fe0.2O3−δ membrane has better oxygen permeation flux than the pristine Ba0.5Sr0.5Co0.8Fe0.2O3−δ membrane. In summary, it has been demonstrated that the surface morphology is an important factor in determining the oxygen permeation fluxes through Ba0.5Sr0.5Co0.8Fe0.2O3−δ membrane under mixed-control conditions.
Similar content being viewed by others
References
B. C. H. Steel, Mater. Sci. Eng., B, 13, 79 (1992).
S.-T. Hwang, Korean J. Chem. Eng., 18, 775 (2001).
Z. Shao, W. Yang, Y. Cong, H. Dong, J. Tong and G. Xiong, J. Membr. Sci., 172, 177 (2000).
H. Wang, Y. Cong and W. Yang, J. Membr. Sci., 210, 259 (2002).
H. Wang, R. Wang, D. T. Liang and W. Yang, J. Membr. Sci., 243, 405 (2004).
S. B. Adler, Chem. Rev., 104, 4791 (2004).
Z. Shao and S. M. Haile, Nature, 431, 170 (2004).
E. Magnone, J. Fuel Cell Sci. Technol., 7, 064001 (2010).
L. Ge, W. Zhou, R. Ran, S. Liu, Z. Shao, W. Jin and N. Xu, J. Membr. Sci., 306, 318 (2007).
J. Sunarso, S. Baumann, J.M. Serra, W.A. Meulenberg, S. Liu, Y. S. Lin and J. C. Diniz da Costa, J. Membr. Sci., 320, 13 (2008).
R.Y. Moydinov, M. N. Popova and A. R. Kaul, Doklady Chemistry, 402, 88 (2005).
H. J.M. Bouwmeester, H. Kruidhof and A. J. Burggraaf, Solid State Ionics, 72, 185 (1994).
C. Ftikos, S. Carter and B. C. H. Steele, J. Eur. Ceram. Soc., 12, 79 (1993).
E. Bucher, A. Egger, P. Ried, W. Sitte and P. Holtappels, Solid State Ionics, 179, 1032 (2008).
S. Carter, A. Selcuk, R. J. Chater, J. Kajda, J.A. Kilner and B. C.H. Steele, Solid State Ionics, 53–56, 597 (1992).
E. Girdauskaite, H. Ullmann, V.V. Vashook, U. Guth, G. B. Caraman, E. Bucher and W. Sitte, Solid State Ionics, 179, 385 (2008).
A. Ghadimi, M. A. Alaee, A. Behrouzifar, A.A. Asadi and T. Mohammadi, Desalination, DOI:10.1016/j.desal.2010.11.022 (2010).
W. K. Hong and G.M. Choi, J. Membr. Sci., 346, 353 (2010).
Z. Chen, R. Ran, Z. Shao, H. Yu, J. C. Diniz da Costa and S. Liu, Ceram. Int., 35, 2455 (2009).
Q. Jiang, K. J. Nordheden and S. M. Stagg-Williams, J. Membr. Sci., DOI:10.1016/j.memsci.2010.11.073 (2010).
J. H. Park, J. P. Kim and S. H. Son, Greenhouse Gas Control Technologies 9, Proceedings of the 9th International Conference on Greenhouse Gas Control Technologies (GHGT-9), 16–20 November 2008, Washington DC, USA, Energy Procedia, 1, 369 (2009).
E. Magnone, M. Miyayama and E. Traversa, J. Electrochem. Soc., 156, B1059 (2009).
H. Lu, Y. Cong and W. S. Yang, Solid State Ionics, 177, 595 (2006).
Z. Shen, P. Lu, G. Yan and X. Hu, Mater. Lett., 64, 980 (2010).
P. Zeng, Z. Chen, W. Zhou, H. Gu, Z. Shao and S. Liu, J. Membr. Sci., 291, 148 (2007).
S. Engels, F. Beggel, M. Modigell and H. Stadler, J. Membr. Sci., 359, 93 (2010).
Z. Yáng, A. S. Harvey and L. J. Gauckler, Scr. Mater., 61, 1083 (2009).
K. Nomura, Z. Homonnay, G. Juhasz, A. Vertes, H. Donen and T.T. s. Sawada, Hyperfine Interact., 139–140, 297 (2002).
Q. Yang and Y. S. Lin, Ind. Eng. Chem. Res., 45, 6302 (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J.H., Magnone, E., Kim, J.P. et al. Oxygen permeation performance of Ba0.5Sr0.5Co0.8Fe0.2O3−δ membrane after surface modification. Korean J. Chem. Eng. 29, 235–242 (2012). https://doi.org/10.1007/s11814-011-0153-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-011-0153-y