Abstract
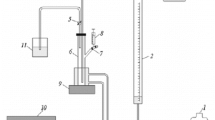
3 gaseous mixtures of CO2, SO2, and NO2 were simultaneously absorbed into 1, 8-diamino-p-menthane (DAM) in a stirred, semi-batch tank with a planar, gas-liquid interface within a range of 0–2.0 kmol/m3 of DAM, 0.05–0.3 atm of CO2, 0.0025–0.04 atm of SO2, and 298.15–323.15 K at a fixed NO2 of 0.001 atm to measure their total molar fluxes. Diffusivity and Henry constants of CO2, SO2, and NO2 were obtained using the reference data, measured by N2O analogy. The mass transfer coefficient of each gas, needed to obtain the absorption rate without a chemical reaction, was modified with viscosity of aqueous DAM solution. In CO2-SO2-NO2-DAM system accompanied by first-order reaction with respect to CO2 and instantaneous reactions with respect to SO2 and NO2, the enhancement factors of CO2 and SO2 were obtained by using an approximate solution of mass balances consisting of reaction regimes of two gases, one of which reacts instantaneously, and then, the enhancement factor of NO2 by comparing the instantaneous rates of SO2 and NO2. The observed values of the molar flux approached to the calculated values very well.
Similar content being viewed by others
References
M. Aresta, Carbon dioxide recovery and utilization, Kluwer Academic Pub., Boston (2003).
M. Caplow, J. Am. Chem. Soc., 90, 6795 (1968).
P. V. Danckwerts, Chem. Eng. Sci., 34, 443 (1979).
E. F. da Silva and H. F. Sendsen, Ind. Eng. Chem. Res., 43, 3413 (2004).
T. Mimura, T. Suda, A. Honda and H. Kumazawa, Chem. Eng. Commun., 170, 245 (1998).
J. Stein, M. Kind and E. Schlunder, Chem. Eng. J., 86, 17 (2002).
S.H. Jung, G. T. Jeong, G.Y. Lee, J. M. Cha and D. H. Park, Korean J. Chem. Eng., 24, 1064 (2007).
S. Ebrahimi, C. Picioreanu, R. Kleerebezem, J. J. Heijnen and M. C. M. van Loosdrecht, Chem. Eng. Sci., 58, 3589 (2003).
S. Colle, J. Vanerschuren and D. Thomas, Chem. Eng. Process, 43, 1397 (2004).
J. Xia, B. Rumpf and G. Maurer, Ind. Eng. Chem. Res., 38, 1149 (1999).
M. H. H. an Dam, A. S. Lamine, D. Roizard, P. Lochon and P. Roizard, Ind. Eng. Chem. Res., 36, 4628 (1997).
D. Nagel, R. de Kermadec, H.G. Lintz, C. Roizard and F. Lapicque, Chem. Eng. Sci., 57, 4883 (2002).
P.V. Danckwerts, Gas-liquid reactions, McGraw-Hill, New York (1970).
K.G. Denbigh and A. J. Prince, J. Am. Chem. Soc., 69, 790 (1947).
P. Gray and A. D. Yoffe, Chem. Rev., 55, 1069 (1955).
J. J. Carberry, Chem. Eng. Sci., 9, 189 (1959).
P.G. Caudle and K.G. Denbigh, Trans. Faraday, Soc., 49, 39 (1959).
M. M. Wendel and R. L. Pigford, J. Am. Chem. Soc., 4, 249 (1958).
M. P. Ho and G. E. Klinzing, Can J. Chem. Eng., 64, 243 (1986).
E. Sada, H. Kumazawa and Y. Yoshikawa, J. Am. Chem. Soc., 34, 1215 (1988).
E.Y. Kenig, R. Schneider and A. Gorak, Chem. Eng. Sci., 54, 5195 (1999).
S.W. Park, D.W. Park, K. J. Oh and S. S. Kim, Sep. Sci. Technol., 44, 543 (2009).
K. S. Hwang, D.W. Kim, S.W. Park, D.W. Park, K. J. Oh and S. S. Kim, Sep. Sci. Technol., 44, 3888 (2009).
K. J. Oh, S. S. Kim and S.W. Park, Sep. Sci. Technol., To be accepted (2010).
L. A. Goetter and R. L. Pigford, J. Am. Chem. Soc., 17, 793 (1971).
H. Hikita, S. Asai and H. Ishikawa, Chem. Eng., J., 18, 169 (1979).
K. J. Oh, Y. S. Choi, S. S. Kim and S.W. Park, Korean J. Chem. Eng., To be accepted (2010).
J. B. Seo, S. B. Jeon, W. J. Choi, J.W. Kim, G. H. Lee and K. J. Oh, Korean J. Chem. Eng., 28, 170 (2011).
L. K. Daraiswany and M. M. Sharma, Heterogeneous reaction: Analysis, example and reactor design, Wiley, New York (1984).
W. Yu, G. Astarita and D.W. Savage, Chem. Eng. Sci., 40, 1585 (1985).
G. F. Versteeg and W. P. M. van Swaaij, J. Chem. Eng. Data, 33, 29 (1988).
A. K. Saha, S. S. Bandyopadhyay and A.K. Biswas, J. Chem. Eng. Data, 38,78 (1993).
W. Pasiuk-Bronikowska and K. J. Rudzinski, Chem. Eng. Sci., 46, 2281 (1991).
F. T. Shadid and D. Handley, Chem. Eng. Res. Dev., 67, 185 (1989).
E. L. Cussler, Diffusion, Cambridge University Press, New York (1984).
G. Carta and R. L. Pigford, Ind. Eng. Chem. Fundam., 22, 329 (1983).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, KJ., Min, BM., Kim, SS. et al. Simultaneous absorption of carbon dioxide, sulfur dioxide, and nitrogen dioxide into aqueous 1, 8-diamino-p-menthane. Korean J. Chem. Eng. 28, 1754–1760 (2011). https://doi.org/10.1007/s11814-011-0025-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-011-0025-5