Abstract
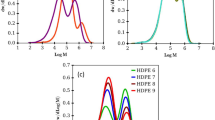
Several ethylene homopolymers and ethene/ α-olefin-copolymers with crystallinities ranging between 85 and 12% were characterized by dynamic-mechanical measurements. The occurring relaxations were correlated to the crystallinity of the polymeric materials and to morphology. The α-relaxation, being attributed to interlamellar shear, was found to be around 60 °C with activation energies of about 120 kJ/mol in samples with more than 42% crystallinity. The β-transition shows a much greater variety among the different samples characterized. Its relaxation temperatures vary between −40 and 10 °C with activation energies between 200 and 400 kJ/mol. The α- and β-relaxation of several quenched samples with crystallinities between 63 and 42% were found to overlap, thus producing bimodal maxima and different activation energies from the Arrhenius plots. A separation of these overlapping relaxations was only possible by measuring the relaxations over a frequency range of more than three orders of magnitude.
Similar content being viewed by others
References
S. Bensason, S. Nazarenko, S. Chum, A. Hiltner and E. Baer, Polymer, 38, 3513 (1997).
G. Hartwig, Polymer Properties at Room and Cryogenic Temperatures, New Yorkm Plenum Press (1994).
K. H. Nitta and A. Tanaka, Polymer, 42, 1219 (2001).
R. H. Boyd, Polymer, 26, 1123 (1985).
R. H. Boyd, Polymer, 26, 323 (1985).
R. Popli, M. Glotin and L. Mandelkern, J. Polym. Sci. Part B: Polym. Phys., 407 (1983).
F. J. Stadler, J. Kaschta and H. Münstedt, Polymer, 46, 10311, DOI: 10.1016/j.polymer.2005.07.099 (2005).
J. Liu, F. Zhang, F. Xie, B. Du, Q. Fu and T. He, Polymer, 42, 5449 (2001).
F. J. Stadler, T. Takahashi and K. Yonetake, e-Polymers, 40, (2009).
F. J. Stadler, T. Takahashi and K. Yonetake, e-Polymers, 41, (2009).
R. O. Sirotkin and N.W. Brooks, Polymer, 42, 9801 (2001).
R.G. Matthews, A. P. Unwin, I. M. Ward and G. Capaccio, Journal of Macromolecular Science-Physics, B38, 1–2, 123–143 (1999).
L. Mandelkern, The crystalline state, 2nd Ed., Chap. 4. Washington DC, ACS (1993).
F. J. Stadler and H. Münstedt, J. Rheology, 52, 697, DOI: 10.1122/1.2892039 (2008).
F. J. Stadler, C. Piel, J. Kaschta, S. Rulhoff, W. Kaminsky and H. Münstedt, Rheologica Acta, 45, 755, DOI: 10.1007/s00397-005- 0042-6 (2006).
F. J. Stadler, C. Piel, W. Kaminsky and H. Münstedt, Macromolecular Symposia, 236, 209, DOI: 10.1002/masy.200650426 (2006).
C. Gabriel and H. Münstedt, Rheologica Acta, 41, 232–244 (2002).
C. Piel, F. J. Stadler, J. Kaschta, S. Rulhoff, H. Münstedt and W. Kaminsky, Macromol. Chem. Phys., 207, 26, DOI: 10.1002/macp.200500321 (2006).
F. J. Stadler, C. Piel, K. Klimke, J. Kaschta, M. Parkinson, M. Wilhelm, W. Kaminsky and H. Münstedt, Macromolecules, 39, 1474, DOI: 10.1021/ma0514018 (2006).
W.W. Graessley and J. Roovers, Macromolecules, 12, 959 (1979).
J. Roovers and W.W. Graessley, Macromolecules, 14, 766 (1981).
R. Godehardt, S. Rudolph, W. Lebek, S. Goerlitz, R. Adhikari, E. Allert, J. Giesemann and G. H. Michler, J. Macromol. Sci. Phys., B38, 5–6, 817–835 (1999).
R. Adhikari, R. Godehardt, W. Lebek, S. Frangov, G. H. Michler, H. Radusch and F. J. B. Calleja, Polym. Adv. Technol., 16, 2–3, 156–166 (2005).
G. Rojas, E. B. Berda and K. B. Wagener, Polymer, 49, 13–14, 2985–2995, DOI 10.1016/j.polymer.2008.03.029 (2008).
S. Głowinkowski, M. Makrocka-Rydzyk, S. Wanke and S. Jurga, European Polym. J., 38, 961 (2002).
D. Mäder, J. Heinemann, P. Walter and R. Mülhaupt, Macromolecules, 33, 1254 (2000).
P. Starck and B. Löfgren, European Polym. J., 38, 97 (2002).
J. J. Dechter, D. E. Axelson, A. Dekmezian, M. Glotin and L. Mandelkern, J. Polym. Sci. Part B: Polym. Phys., 20, 641 (1982).
The interfacial regime is believed to be a rather thin layer on the border between the crystal lamellae and the amorphous regime.
J. A. Resch, F. J. Stadler, J. Kaschta and H. Münstedt, Macromolecules, 42, 5676, DOI: 10.1021/ma9008719 (2009).
U. Keßner, H. Münstedt, J. Kaschta, F. J. Stadler, C. S. Le Duff and X. Drooghaag, Macromolecules, In press, DOI: 10.1021/ma100705f (2010).
X. Chen, F. J. Stadler, H. Münstedt and R.G. Larson, Journal of Rheology, 54, 393, DOI: 10.1122/1.3305721 (2010).
F. J. Stadler, J. Kaschta and H. Münstedt, Macromolecules, 41, 1328, DOI: 10.1021/ma702367a (2008).
F. J. Stadler, A. Nishioka, J. Stange, K. Koyama and H. Münstedt, Rheologica Acta, 46, 1003, DOI: 10.1007/s00397-007-0190-y (2007).
F. J. Stadler, C. Gabriel and H. Münstedt, Macromol. Chem. Phys., 208, 2449, DOI: 10.1002/macp.200700267 (2007).
In other words, the material behaves thermorheologically complex, as several processes with different activation energies overlap each other. Thus, no master curve can be constructed, but instead a discussion about the relaxation time dependent activation energy would have to be conducted [30,33]. However, this complicated method does not have to be conducted, as E″ is a clearly separable peak, whose activation energy can, therefore, be determined with relative ease from the peak temperature.
Determined as the difference quotient.
C. Piel, P. Starck, J.V. Seppälä and W. Kaminsky, J. Polym. Sci. Part A: Polym. Chem., 44, 1600 (2006).
This value was adopted, as it is approximately the mean of the crystallinity of the neighboring samples.
C. Piel, Polymerizations of Ethene and Ethene-co-alpha-Olefin: Investigations on Short- and Long-Chain Branching and Structure Property Relationships. Department of Technical and Macromolecular Chemistry, Vol. Ph. D. Hamburg: University of Hamburg (2005).
C. Piel, K. Scharlach and W. Kaminsky, Macromolecular Symposia, 226, 25 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to Professor Ki Ju Kim for celebrating his retirement from Division of Chemical Engineering of Chonbuk National University
Rights and permissions
About this article
Cite this article
Stadler, F.J. Dynamic-mechanical behavior of polyethylenes and ethene/α-olefin-copolymers: Part II. α- and β-relaxation. Korean J. Chem. Eng. 28, 954–963 (2011). https://doi.org/10.1007/s11814-010-0411-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-010-0411-4