Abstract
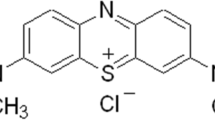
An ion exchanger with carboxyl groups as active sites was prepared by activating sawdust with epichlorohydrin, followed by coupling the epoxy-activated sawdust with aspartic acid. The optimal sorption condition, sorption capacity, kinetics and thermodynamics of basic dyes on sawdust ion exchanger (SIE) from aqueous solution were investigated in a batch system. Two basic dyes, methylene blue (MB) and crystal violet (CV), were selected as sorbates. The optimal pH value of MB and CV solutions for favorable sorption was pH 4 and above. The removal ratios of MB and CV on SIE increased with increasing sorbent dose but decreased with increasing dye concentration. The isothermal data of MB and CV sorbed on SIE correlated basically with the Langmuir model. The maximum sorption capacity (Q m ) of SIE for MB and CV was 222.22 and 232.56 mg/g, respectively. The sorption equilibriums of MB and CV on SIE were reached at about 9 h, and the sorption processes could be described by the pseudo-second-order kinetic model. The thermodynamic study indicated that the sorptions of MB and CV on SIE were spontaneous and endothermic at the predetermined temperatures. High temperatures were favorable for the sorption processes.
Similar content being viewed by others
References
C. I. Pearce, J. R. Lloyd and J. T. Guthrie, Dyes Pigments, 58, 179 (2003).
G. McMullan, C. Meehan, A. Conneely, N. Kirby, T. Robinson, P. Nigam, I. M. Banat, R. Marchant and W. F. Smyth, Appl. Microbiol. Biotechnol., 56, 81 (2001).
G. McKay, M. S. Otterburn and D. A. Aga, Water Air Soil Pollut., 24, 307 (1985).
A. R. Gregory, S. Elliot and P. Kluge, J. Appl. Toxicol., 1, 308 (1991).
B. C. Kim, Y.H. Kim and T. Yamamoto, Korean J. Chem. Eng., 25, 1140 (2008).
S. J. Allen, Q. Gan, R. Matthews and P.A. Johnson, Bioresour. Technol., 88, 143 (2003).
V. Vadivelan and K. V. Kumar, J. Colloid Interf. Sci., 286, 90 (2005).
R. Gong, M. Li, C. Yang, Y. Sun and J. Chen, J. Hazard. Mater., 121, 247 (2005).
Y. Bulut and H. A. Aydin, Desalination, 194, 259 (2006).
M. T. Sulak, E. Demirbas and M. Kobya, Bioresour. Technol., 98, 2590 (2007).
K. V. Kumar, Dyes Pigments, 74, 595 (2007).
J. F. Osma, V. Saravia, J. L. Toca-Herrera and S. R. Couto, J. Hazard. Mater., 147, 900 (2007).
B. H. Hameed and M. I. El-Khaiary J. Hazard. Mater., 154, 639 (2008).
B.H. Hameed, D.K. Mahmoud and A. L. Ahmad, Colloid. Surface. A, 316, 78 (2008).
F.A. Pavan, E. C. Lima, S. L. P. Dias and A.C. Mazzocato, J. Hazard. Mater., 150, 703 (2008).
V. Marchetti, P. Gérardin and B. Loubinoux, Holz Roh Werkst., 58, 53 (2000).
R. Gong, Y. Jin, F. Chen, J. Chen and Z. Liu, J. Hazard. Mater., 137, 865 (2006).
S. T. Ong, C. K. Lee and Z. Zainal, Bioresour. Technol., 98, 2792 (2007).
M.V. Sureshkumar and C. Namasivayam, Colloid. Surface. A, 317, 277 (2008).
A. Di budak, S. Bekta, S. Patır, Ő Genç and A. Denizli, Sep. Purif. Technol., 26, 273 (2002).
S.C. Gupta, P. Dass, P. Sharma, A.V. Singh and S. Gupta, Desalination, 143, 141 (2002).
K. Oshita, M. Oshima, Y. Gao, K.-H. Lee and S. Motomizu, Anal. Chim. Acta, 480, 239 (2003).
Y.-L. Lei, D.-Q. Lin, S.-J. Yao and Z.-Q. Zhu, React. Funct. Polym., 62, 169 (2005).
S. Chakraborty, S. De, S. DasGupta, J.K. Basu, Chemosphere, 58, 1079 (2005).
V. P. Vinod and T. S. Anirudhan, Water Air Soil Pollut., 150, 193 (2003).
K. Porkodi and K.V. Kumar, J. Hazard. Mater., 143, 311 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gong, R., Li, N., Ni, S. et al. Preparation of sawdust functionalized with aspartic acid and its sorption capacity, kinetics and thermodynamics for basic dyes. Korean J. Chem. Eng. 27, 1560–1564 (2010). https://doi.org/10.1007/s11814-010-0249-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-010-0249-9