Abstract
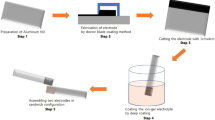
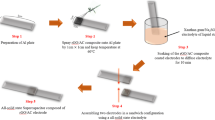
A hybrid asymmetrical super capacitor has been fabricated based on p-doped poly(aniline-co-m-anilicacid) and activated carbon coated on SS electrodes. The characterization of material, electrode and performance of the super capacitor has been studied by FTIR, Cyclic Voltammetry, TGA/DTA, AC Impedance spectroscopy, and galvanostatic charge-discharge tests. The super capacitor showed a maximum specific capacitance of 102 F/g at a scan rate of 10 mV/s. The normalized active-reactive power behavior of the capacitor has been determined. The time constant calculated for the super capacitor is 6 milliseconds, indicating the suitability of the system for efficient use at low frequency range.
Similar content being viewed by others
References
P. Croce, S. Passerini and B. Scrosati, J. Electrochem. Soc., 141, 1405 (1994).
J. Osaka, S. Komaba, N. Fujihana, J. Momma and N. T. Laneco, J. Electrochem. Soc., 144, 742 (1997).
G. P. Kitteson, H. S. White and M. S. Wrighton, J. Am. Chem. Soc., 106, 7389 (1984).
B. R. Mattes, H. Wang, D. Yang, Y. T. Zhu, W. R. Blamenthal and M. F. Hundley, Synth. Met., 45, 84 (1997).
A. Rudge, J. Davey, J. Raistrick and S. Gottesteid, J. Power Sources, 47, 89 (1994).
J. Libent, J.W. Bredas and A. J. Esptein, Phys. Rev. B., 51, 5711 (1995).
M.Y. Hua, G.W. Hwang, Y.H. Chuang, S.A. Chen and R.Y. Tsai, Macromolecules, 33, 6235 (2000).
A.A. Karyakin, A.K. Strakhova and A.K. Yatsimirsky, J. Electroanal. Chem., 259, 371 (1994).
W. Shenglong, W. Fosong and G. Xiabhui, Synth. Met., 16, 99 (1998).
M. Leclerc, J. Guay and L. H. Dao, J. Electroanal. Chem., 21, 251 (1988).
D. Macinnes and B. L. Funt, Synth. Met., 25, 235 (1998).
W.A. T. Gazotti, J. Matecio and M.A. Depaoli, Electrochim. Acta, 43, 457 (1998).
X.H. Wang, L. X. Wang, X. P. Jing and F. S. Wang, Synth. Met., 69, 335 (1995).
M. Ranger and M. Leclerc, Synth. Met., 84, 85 (1997).
M.A. Christopher and C. Brett Thiemann, J. Electroanal. Chem., 538, 215 (2002).
K. Ryu, K. Kim, N. Park, Y. Park and S. Chang, J. Power Sources, 103, 305 (2002).
J. H. Park and O. Park, J. Power Sources, 111, 185 (2002).
D.W. Hatchett, N. Jsowicz and J. Janata, J. Electrochem. Soc., 146, 4535 (1999).
K. S. Ryu, K. M. Kim, N.-G. Park, Y. J. Park and S.H. Chang, J. Power Sources, 103, 305 (2002).
A. Clemete, S. Panero, E. Spila and B. Scrosati, Solid State Ionics, 85, 273 (1996).
C. Arbizzani, M. Mastragostino and F. Soavi, J. Power Sources, 100, 164 (2001).
K. S. Ryu, Y.-G. Lee, Y.-S. Hong, Y. J. Park, X. Wu, K.M. Kim, M.G. Kang, N.-G. Park and S.H. Chang, Electrochim. Acta, 50, 843 (2004).
K. I. Seo and I. J. Chung, Polym., 41, 4491 (2004).
F. Tranvan, S. Garreau, G. Louarn, G. Froyer and C. Chevrot, J. Mater Chem., 11, 1378 (2001).
D.W. Hatchett, M. Jsowicz and J. Janata, J. Electrochem. Soc., 146(12), 4535 (1999).
Y. Wei and K. F. Hsueh, J. Polym. Sci., 27, 4351 (1989).
R. Ansari, W. E. Price and G.G. Wallace, React. Funct. Polym., 56, 141 (2003).
T. Hagiwara, M. Yamaura and K. Iwata, Synth. Met., 25, 243 (1988).
B. E. Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, Kluwer Academic Publishers, Plenum press, New York (1999).
D. Qu and H. Shi, J. Power Sources, 74, 99 (1998).
H.K. Song, Y.H. Jung, K.H. Lee and L.H. Dao, Electrochim. Acta, 44, 3513 (1999).
H. Keiser, K. D. Beccu and M. A. Gutjahr, Electrochim Acta, 12, 539 (1976).
P. L. Taberna, P. Simon and J. F. Fauvarque, J. Electrochem. Soc., 150(3), A292 (2003).
J. P. Zheng, J. Huang and T.R. Jow, J. Electrochem. Soc., 144(6), 2026 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selvakumar, M., Pitchumani, S. Hybrid supercapacitor based on poly(aniline-co-m-anilicacid) and activated carbon in non-aqueous electrolyte. Korean J. Chem. Eng. 27, 977–982 (2010). https://doi.org/10.1007/s11814-010-0120-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-010-0120-z