Abstract
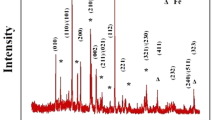
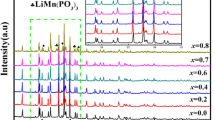
A new type of Li1−x Fe0.8Ni0.2O2-Li x MnO2 (Mn/(Fe+Ni+Mn)=0.8) material was synthesized at 350 °C in an air atmosphere by a solid-state reaction. The material had an XRD pattern that closely resembled that of the original Li1−x FeO2-Li x MnO2 ((Fe+Ni+Mn)=0.8) with much reduced impurity peaks. It was composed of many large particles of about 500–600 nm and small particles of about 100–200 nm, which were distributed among the larger particles. The Li/Li1−x Fe0.8Ni0.2O2-Li x MnO2 cell showed a high initial discharge capacity above 192 mAh/g, which was higher than that of the parent Li/Li1−x FeO2-Li x MnO2 (186 mAh/g). This cell exhibited not only a typical voltage plateau in the 2.8 V region, but also an excellent cycle retention rate (96%) up to 45 cycles. We suggest a unique role of doped nickel ion in the Li/Li1−x Fe0.8Ni0.2O2-Li x MnO2 cell, which results in the increased initial discharge capacity from the redox reaction of Ni2+/Ni3+ between 2.0 and 1.5 V region.
Similar content being viewed by others
References
K. Mizushima, P. C. Jones, P. J. Wiseman and J. B. Goodenough, Mater. Res. Bull., 15, 783 (1980).
E. Rossen, J.N. Reimers and J.R. Dahn, Solid State Ionics, 62, 53 (1993).
T. Ohzuku, A. Ueda, M. Nagayama, Y. Iwakoshi and H. Komori, Electrochim. Acta, 38, 1159 (1993).
D. H. Jang, Y. J. Shin and S.M. Oh, J. Electrochem. Soc., 143, 2204 (1996).
Y. Xia, Y. Zhou and M. Yoshio, J. Electrochem. Soc., 144, 2593 (1997).
Y. Xia and M. Yoshio, J. Power Sources, 57, 125 (1995).
R. Kanno, T. Shirane, Y. Kawamoto, Y. Takeda, M. Takano, M. Ohashi and Y. Yamaguchi, J. Electrochem. Soc., 143, 2435 (1996).
T. Shirane, R. Kanno, Y. Kawamoto, Y. Takeda, M. Takano, T. Kamiyama and F. Izumi, Solid State Ionics, 79, 227 (1995).
M. Tabuchi, K. Ado, H. Sakaebe, C. Masquelier, H. Kageyama and O. Nakamura, Solid State Ionics, 79, 220 (1995).
M. Tabuchi, C. Masquelier, T. Takeuchi, K. Ado, I. Matsubara, T. Shirane, R. Kanno, S. Tsutsui, S. Nasu, H. Sakaebe and O. Nakamura, Solid State Ionics, 90, 129 (1996).
K. Ado, M. Tabuchi, H. Kobayashi, H. Kageyama, O. Nakamura, Y. Inaba, R. Kanno, M. Takagi and Y. Takeda, J. Electrochem. Soc., 144, L177 (1997).
M. Tabuchi, K. Ado, H. Kobayashi, I. Matsubara, H. Kageyama, M. Wakita, S. Tsutsui, S. Nasu, Y. Takeda, C. Masqulier, A. Hirano and R. Kanno, J. Solid State Chem., 141, 554 (1998).
M. Tabuchi, S. Tsutsui, C. Masquelier, R. Kanno, K. Ado, I. Matsubara, S. Nasu and H. Kageyama, J. Solid State Chem., 140, 159 (1998).
Y. Sakurai, H. Arai, S. Okada and J. Yamaki, J. Power Sources, 68, 711 (1997).
Y. Sakurai, H. Arai and J. Yamaki, Solid State Ionics, 113–115, 29 (1998).
Y. S. Lee, S. Sato, M. Tabuchi, C. S. Yoon, Y.K. Sun, K. Kobayakawa and Y. Sato, Electrochem. Comm., 5, 549 (2003).
Y. S. Lee, S. Sato, Y.K. Sun, K. Kobayakawa and Y. Sato, Electrochem. Comm., 5, 359 (2003).
Y. S. Lee, C. S. Yoon, Y. K. Sun, K. Kobayakawa and Y. Sato, Electrochem. Comm., 4, 727 (2002).
Y. S. Lee and M. Yoshio, Electrochem. Solid-State Lett., 4(10), A166 (2001).
Y. S. Lee, C. S. Yoon, Y. K. Sun and M. Yoshio, Electrochem. Solid-State Lett., 5(1), A1 (2002).
S.G. Youn, I.H. Lee, C. S. Yoon, C.K. Kim, Y.K. Sun, Y. S. Lee and M. Yoshio, J. Power Sources, 108(1–2), 97 ( 2002).
Y. Ito, Y. Idemoto, Y. Tsunoda and N. Koura, J. Power Sources, 119–121, 733 (2003).
H. Ikuta, K. Takanaka and M. Wakihara, Electrochim. Acta, 414(2), 227 (2004).
S. H. Park, K. S. Park, K. S. Nahm, Y. K. Sun, Y. S. Lee and M. Yoshio, Electrochim. Acta, 47, 1721 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, GJ., Heo, JB., Cho, SH. et al. Preparation, electrochemical properties, and cycle mechanism of Li1−x Fe0.8Ni0.2O2-Li x MnO2 (Mn/(Fe+Ni+Mn)=0.8) material. Korean J. Chem. Eng. 25, 1389–1396 (2008). https://doi.org/10.1007/s11814-008-0228-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-008-0228-6