Abstract
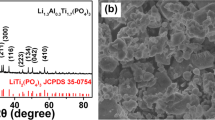
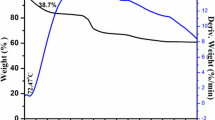
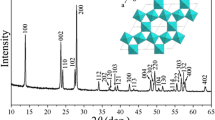
Recently, lithium titanium oxide material has gained renewed interest in electrodes for lithium ion rechargeable batteries. We investigated the influence of excess Li on the structural characteristics of lithium titanium oxide synthesized by the conventional powder calcination method, considering the potential for mass production. The lithium excess ratio is controlled by using different weight of Li2CO3 powder during calcination. X-ray diffraction (XRD) measurement for the synthesized powder showed that the lithium titanium oxide material with excess lithium content had a spinel crystal structure as well as a different crystal one. In addition, high resolution transmission electron microscopy (HRTEM) and field emission scanning electron microscopy (FESEM) measurement revealed that the lithium titanium oxide powders with a lithium excess ratio of 5–20% exhibited a two phase formation. Inductively coupled plasma — atomic emission spectrometer (ICP-AES) and energy dispersive x-ray spectroscopy (EDX) measurements were used to analyze composition of the lithium titanium oxide powder. These results suggested that the conventional calcination method, considering the potential for mass production, formed two phases according to the Li excess amount in initial raw materials.
Similar content being viewed by others
References
Colbow, K.M., Dahn, J. R. and Haering, R. R., “Structure and electrochemistry of the spinel oxides LiTi2O4 and Li4/3Ti5/3O4,” J. Power Sources, 26, 397 (1989).
Deschanvers, A., Raveau, B. and Sekkai, Z., “Mise en évidence et etude cristallographique d’une nouvelle solution solide de type spinelle Li1+x Ti2−x O4 0≤x≤0.33,” Mat. Res. Bull., 6, 699 (1971).
Doh, C.-H., Jin, B.-S., Lim, J.-H. and Moon, S.-I., “Electrochemical characteristics of lithium transition-metal oxide as an anode material in a lithium secondary battery,” Korean J. Chem. Eng., 19, 749 (2002).
Ferg, E., Gummov, R. J., de Kock, A. and Thakeray, M. M., “Spinel anodes for lithium-ion batteries,” J. Electrochem. Soc., 141, L147 (1994).
Jovic, N., Antic, B., Kremenovic, A., Spasojevic-de Bire and Spasojevic, A. V., “Cation ordering and order-disorder phase transition in Cosubstituted Li4Ti5O12 spinels,” Phy. Stat. Sol., (a) 198, 18 (2003).
Ohzuku, T., Uedo, A. and Yamamoto, N., “Zero-strain insertion material of Li[Li1/3Ti5/3O1/4] for rechargeable lithium cells,” J. Electrochem. Soc., 142, 1431 (1995).
Park, K. S., Cho, M. H., Jin, S. J., Song, C. H. and Nahm, K. S., “Structural and electrochemical characteristics of Li0.7[Li1/6Mn5/6]O2 synthesized using sol-gel method,” Korean J. Chem. Eng., 22, 46 (2005).
Prosini, P. P., Mancini, R., Pertucci, L., Contini, V. and Villano, P., “Li4Ti5O12 as anode in all-solid-state, plastic, lithium-ion batteries for lowpower applications,” Solid State Ionics, 144, 185 (2001).
Rho, Y. H., Kanamura, K., Fujisaki, M., Hamagami, J.-i., Suda, S.-i. and Umegaki, T., “Preparation of Li4Ti5O12 and LiCoO2 thin film electrodes from precursors obtained by sol-gel method,” Solid State Ionics, 151, 151 (2002).
Scharner, S., Weppner, W. and Schmid-Beurmann, P., “Evidence of two-phase formation upon lithium insertion into the Li1.33Ti1.67O4 spinel,” J. Electrochem. Soc., 146, 857 (1999).
Sun, Y.-K., Kim, D.-W., Jin, S.-H., Hyung, Y.-E., Moon, S.-I. and Park, D.-K., “Synthesis and cycling behavior of LiMn2O4 cathode materials prepared by glycine-assisted sol-gel method for lithium secondary batteries,” Korean J. Chem. Eng., 15, 64 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S.H., Lee, K.H., Seong, B.S. et al. Synthesis and structural properties of lithium titanium oxide powder. Korean J. Chem. Eng. 23, 961–964 (2006). https://doi.org/10.1007/s11814-006-0015-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11814-006-0015-1