Abstract
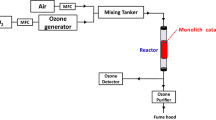
Both flat and corrugated wire mesh sheets were coated with aluminum powder by using electrophoretic deposition (EPD) method. Controlled thermal sintering of coated samples yielded uniform porous aluminum layer with a thickness of 100 μm that was attached firmly on the wire meshes. Subsequent controlled calcination formed a finite thickness of Al2O3 layer on the outer surface of each deposited aluminum particles, which resulted in the formation of Al2O3/Al double-layered composite particles that were attached firmly on the wire surface to form a certain thickness of porous layer. A rectangular-shaped wire-mesh honeycomb (WMH) module with triangular-shaped channels was manufactured by packing alternately the flat sheet and corrugated sheet of the Al2O3/Al-coated wire meshes. This WMH was further coated with V2O5-MoO3-WO3 catalyst by wash-coating method to be applied for the selective catalytic reduction (SCR) of NO with NH3. With an optimized catalyst loading of 16 wt%, WMH catalyst module shows more than 90% NO conversion at 240 °C and almost complete NO conversion at temperatures higher than 300 °C at GHSV 5,000 h−1. When compared with conventional ceramic honeycomb catalyst, WMH catalyst gives NO conversion higher by 20% due to reduced mass transfer resistance by the existence of three dimensional opening holes in WMH.
Similar content being viewed by others
References
Ahlstrom-Silversand, A. F. and Odenbrand, C.U. I., “Thermally sprayed wire-mesh catalysts for the purification of flue gases from small-scale combustion of bio-fuel Catalyst preparation and activity studies,” Applied Catalysis, A, 153(1–2), 177 (1997).
Amiridis, M. D., Duevel, R.V. and Wachs, I. E., “The effect of metal oxide additives on the activity of V2O5/TiO2 catalysts for the selective catalytic reduction of nitric oxide by ammonia,” Applied Catalysis B: Environmental, 20(2), 111 (1999).
de Boer, M., Huisman, H. M., Mos, R. J. M., Leliveld, R.G., van Dillen, A. J. and Geus, J.W., “Selective oxidation of ammonia to nitrogen over SiO2-supported MoO3 catalysts,” Catalysis Today, 17(1–2), 189 (1993).
Bosch, H., Janssen, F. J. J.G., van den Kerkhof, F. M.G., Oldenziel, J., van Ommen, J.G. and Ross, J.R. H., “The activity of supported vanadium oxide catalysts for the selective reduction of NO with ammonia,” Applied Catalysis, 25, 239 (1986).
Chae, H. J., Nam, I.-S., Ham, S.-W. and Hong, S. B., “Characteristics of vanadia on the surface of V2O5/Ti-PILC catalyst for the reduction of NOx by NH3,” Applied Catalysis B: Environmental, 53(2), 117 (2004).
Chen, J. P. and Yang, R. T., “Mechanism of poisoning of the V2O5/TiO2 catalyst for the reduction of NO by NH3,” Journal of Catalysis, 125(2), 411 (1990).
Choi, H., Ham, S.W., Nam, I. S., Kim, Y.G., Shim, J.H. and Ha, B.H., “NO reduction with ammonia using parallel passage reactor,” HWAHAK KONGHAK, 34, 91 (1996).
Chung, J. H., Shon, B. H., Yoo, K. S., Kim, H. K. and Lee, H. K., “Simultaneous removal of SO2 and NOx by the absorbent from coal fly ash,” HWAHAK KONGHAK, 41, 403 (2003).
Chung, K.-S., Jiang, Z., Gill, B.-S. and Chung, J. S., “Oxidative decomposition of o-dichlorobenzene over V2O5/TiO2 catalyst washcoated onto wire-mesh honeycombs,” Applied Catalysis A: General, 237(1–2), 81 (2002).
Cybulski, A. and Moulijn, J. A. (ed.), Structured catalysts and reactors, Marcel Dekker, Inc. (1998).
Dutoit, D. C. M., Reiche, M. A. and Baiker, A., “Vanadia-silica aerogels. Structure and catalytic properties in selective reduction of NO by NH3,” Applied Catalysis B: Environmental, 13(3–4), 275 (1997).
Handy, B. E., Baiker, A., Schraml-Marth, M. and Wokaun, A., “Vanadia supported on TiO2—SiO2 mixed oxide gels: Structure of the dispersed phase and activity for the selective catalytic reduction of NO with NH3,” Journal of Catalysis, 133(1), 1 (1992).
Handy, B. E., Maciejewski, M. and Baiker, A., “Vanadia, vanadia-titania, and vanadia-titania-silica gels: Structural genesis and catalytic behavior in the reduction of nitric oxide with ammonia,” Journal of Catalysis, 134(1), 75 (1992).
Jiang, Z., Chung, K.-S., Kim, G.-R. and Chung, J. S., “Mass transfer characteristics of wire-mesh honeycomb reactors,” Chemical Engineering Science, 58(7), 1103 (2003).
Kim, B. S., Lee, S.H., Park, Y. T., Ham, S.W., Chae, H. J. and Nam, I. S., “Selective catalytic reduction of NOx by propene over copper-exchanged pillared clays,” Korean J. Chem. Eng., 18, 704 (2001).
Kim, B. T., Lee, H.G., Chun, G. S., Lee, G. J. and Park, H. S., “NOx absorption into aqueous solutions: II. HNO3 and HNO2 concentration produced by NOx absorption for a sieve tray column,” HWAHAK KONGHAK, 25(2), 169 (1987).
Kim, G. R., Jiang, Z. and Chung, J. S., Korean Patent 0336821 (2002).
Lee, I.-Y., Kim, D.-W., Lee, J.-B. and Yoo, K.-O., “A practical scale evaluation of sulfated V2O5/TiO2 catalyst from metatitanic acid for selective catalytic reduction of NO by NH3,” Chemical Engineering Journal, 90(3), 267 (2002).
Lee, H. T. and Rhee, H. K., “Steam tolerance of Fe/ZSM-5 catalyst for the selective catalytic reduction of NOx,” Korean J. Chem. Eng, 19, 574 (2002).
Lee, S. H., Ahn, J. S. and Kim, J. H., “Selective catalytic reduction of NOx catalyst,” News & Information for Chem. Eng., 19(4), 468 (2001).
Lintz, H.-G. and Turek, T., “Intrinsic kinetics of nitric oxide reduction by ammonia on a vanadia-titania catalyst,” Applied Catalysis A: General, 85(1), 13 (1992).
Liuqing, T., Daiqi, Ye. and Hong, L., “Catalytic performance of a novel ceramic-supported vanadium oxide catalyst for NO reduction with NH3,” Catalysis Today, 78(1–4), 159 (2003).
Long, R. Q. and Yang, R. T., “Catalytic performance and characterization of VO2+-exchanged titania-pillared clays for selective catalytic reduction of nitric oxide with ammonia,” Journal of Catalysis, 196(1), 73 (2000).
Lucas, D. and Brown, N. J., “Characterization of the selective reduction of NO by NH3,” Combustion and Flame, 47, 219 (1982).
Park, J. H., Kim, D. J. and Kim, G. S., “Analysis on NOx conversion and particle characteristics in NOx removal by corona discharge,” HWAHAK KONGHAK, 40, 351 (2002).
Piehl, G., Liese, T. and Grünert, W., “Activity, selectivity and durability of VO-ZSM-5 catalysts for the selective catalytic reduction of NO by ammonia,” Catalysis Today, 54(4), 401 (1999).
Rajadhyaksha, R. A., Hausinger, G., Ramstetter, H. Z. and Knöinger, H. S., “Vanadia supported on titania-silica: Physical characterization and activity for the selective reduction on nitric oxide,” Applied Catalysis, 51(1), 67 (1989).
Topsoe, N.Y., Topsoe, H. and Dumesic, J. A, “Vanadia/titania catalysts for selective catalytic reduction (SCR) of nitric-oxide by ammonia: I. Combined temperature-programmed in-situ FTIR and on-line mass-spectroscopy studies,” Journal of Catalysis, 151(1), 226 (1995).
Weng, R.-Y. and Lee, J.-F., “Catalytic performance and active sites determination of niobium oxide promoted vanadia/titania catalysts for selective catalytic reduction of nitric oxide,” Applied Catalysis A: General, 105(1), 41 (1993).
Went, G. T., Leu, L.-J., Rosin, R. R. and Bell, A. T., “The effects of structure on the catalytic activity and selectivity of V2O5/TiO2 for the reduction of NO by NH3,” Journal of Catalysis, 134(2), 492 (1992).
Yang, K. S., Choi, J. and Chung, J. S., “Evaluation of wire-mesh honeycomb containing porous Al/Al2O3 layer for catalytic combustion of ethyl acetate in air,” Catalysis Today, 97(2–3), 27 159 (2004).
Yang, K. S., Jiang, Z. and Chung, J. S., “Electrophoretically Al-coated wire mesh and its application for catalytic oxidation of 1,2-dichlorobenzene,” Surface and Coatings Technology, 168(2–3), 103 (2003).
Yoo, K. S., Lee, J.G., Park, D.G., Chung, M. J., Lee, C. and Shin, J.W., “The selective non-catalytic reduction reaction of NOx using urea solution in a flow reactor,” HWAHAK KONGHAK, 41, 219 (2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, J., Choi, J.S., Yang, K.S. et al. Wire-mesh honeycomb catalysts for selective catalytic reduction of NO with NH3 . Korean J. Chem. Eng. 23, 888–895 (2006). https://doi.org/10.1007/s11814-006-0004-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11814-006-0004-4