Abstract
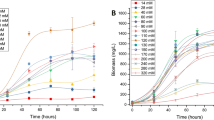
Filamentous microalgae from genus Tribonema are promising sustainable sources of omega-7 palmitoleic acid, but their ability to accumulate this compound varies among species and depends on the initial nitrogen concentration (INC) supply. In this study, the palmitoleic acid accumulation capacities of five Tribonema species were examined under three different INCs to select the alga species with the highest production. Results showed that a high INC was associated with increased palmitoleic acid accumulation but led to decreased biomass concentration in all tested species. In particular, T. minus grown at 18 mmol L−1 INC had the highest palmitoleic acid content (20.72% of dry weight) and productivity (90.88 mg L−1 d−1). The combination of alkali metal freezing precipitation (AMFP) and urea complexation successfully isolated and enriched palmitoleic acid from T. minus and obtained a purity of 80.11% and a yield of 7.39 g(100 g)−1 of algal powder. The compound was identified as (9Z)-hexadecenoic acid (C16:1 ω-7). Antibacterial activity evaluation for the highly concentrated palmitoleic acid (10 mg mL−1) against Streptococcus agalactiae revealed the formation of a 12.10 mm-diameter inhibition zone and the minimum inhibitory concentration of 31.25 µg mL−1, indicating that palmitoleic acid is an effective antibacterial agent. This study is the first to report that palmitoleic acid derived from T. minus can antagonize S. agalactiae, which further broadens the potential application of Tribonema biomass in green aquaculture.
Similar content being viewed by others
References
Abdel-Razek, A. S., El-Naggar, M. E., Allam, A., and Morsy, O. M., 2020. Microbial natural products in drug discovery. Processes, 8(4): 470, DOI: https://doi.org/10.3390/pr8040470.
Akgul, F., 2020. Effects of nitrogen concentration on growth, biomass, and biochemical composition of Desmodesmus communis (E. Hegewald) E. Hegewald. Preparative Biochemistry Biotechnology, 50(1): 98–105, DOI: https://doi.org/10.1080/10826068.2019.1697884.
Betriu, C., Culebras, E., Gómez, M., Rodriguez-Avial, I., Sanchez, B. A., Agreda, M. C., et al., 2003. Erythromycin and clindamycin resistance and telithromycin susceptibility in Streptococcus agalactiae. Antimicrobial Agents and Chemotherapy, 47(3): 1112–1114, DOI: https://doi.org/10.1128/AAC.47.3.1112-1114.2003.
Chen, W. J., Wang, Y., Han, D., Zhu, X. M., Xie, S. Q., Han, D. X., et al., 2019. Two filamentous microalgae as feed ingredients improved flesh quality and enhanced antioxidant capacity and immunity of the gibel carp (Carassius auratus gibelio). Aquaculture Nutrition, 25(5): 1145–1155, DOI: https://doi.org/10.1111/anu.12930.
Chideroli, R. T., Amoroso, N., Mainardi, R. M., Suphoronski, S. A., and Pereira, U. P., 2017. Emergence of a new multidrug-resistant and highly virulent serotype of Streptococcus agalactiae in fish farms from Brazil. Aquaculture, 479: 45–51, DOI: https://doi.org/10.1016/j.aquaculture.2017.05.013.
Chu, W. L., and Phang, S. M., 2019. Bioactive compounds from microalgae and their potential applications as pharmaceuticals and nutraceuticals. In: Grand Challenges in Algae Biotechnology. Hallmann, A., and Rampelotto, P. H., eds., Springer, Cham, 429–469.
Claudia, M., Fabiana, P., Maia, C. F. C., Ribeiro, B. H., and Campos, C. E., 2018. Antimicrobial activity of some essential oils against Streptococcus agalactiae, an important pathogen for fish farming in Brazil. Journal of Essential Oil Research, 30: 388–397, DOI: https://doi.org/10.1080/10412905.2018.1487343.
Deng, L. S., Li, Y. J., Geng, Y., Zheng, L. P., Rehman, T., Zhao, R. X., et al., 2019. Molecular serotyping and antimicrobial susceptibility of Streptococcus agalactiae isolated from fish in China. Aquaculture, 510: 84–89, DOI: https://doi.org/10.1016/j.aquaculture.2019.05.046.
Desbois, A. P., Lebl, T., Yan, L. M., and Smith, V. J., 2008. Isolation and structural characterisation of two antibacterial free fatty acids from the marine diatom, Phaeodactylum tricornutum. Applied Microbiology & Biotechnology, 81: 755–764, DOI: https://doi.org/10.1007/s00253-008-1714-9.
Gao, B. Y., Yang, J., Lei, X. Q., Xia, S., Li, A. F., and Zhang, C. W., 2016. Characterization of cell structural change, growth, lipid accumulation, and pigment profile of a novel oleaginous microalga, Vischeria stellata (Eustigmatophyceae), cultured with different initial nitrate supplies. Journal of Applied Phycology, 28(2): 821–830, DOI: https://doi.org/10.1007/s10811-015-0626-1.
Genilloud, O., 2019. Natural products discovery and potential for new antibiotics. Current Opinion in Microbiology, 51: 81–87, DOI: https://doi.org/10.1016/j.mib.2019.10.012.
Guo, F., Wang, H., Wang, J., Zhou, W. J., Gao, L. L., Dong, Q. Z., et al., 2014. Special biochemical responses to nitrogen deprivation of filamentous oleaginous microalgae Tribonema sp.. Bioresource Technology, 158: 19–24, DOI: https://doi.org/10.1016/j.biortech.2014.01.144.
Hu, W., Fitzgerald, M. A., Topp, B., Alam, M., and Hare, T. J. O., 2019. A review of biological functions, health benefits, and possible de novo biosynthetic pathway of palmitoleic acid in macadamia nuts. Journal of Functional Foods, 62: 103520, DOI: https://doi.org/10.1016/j.jff.2019.103520.
Liu, G., Zhu, J., Chen, K., Yao, H., and Lu, C., 2016. Development of Streptococcus agalactiae vaccines for tilapia. Diseases of Aquatic Organisms, 122(2): 163–170, DOI: https://doi.org/10.3354/dao03084.
Mehari, B., Rediabshiro, M., Chandravanshi, B. S., Combrinck, S., McCrindle, R., and Atlabachew, M., 2019. GC-MS profiling of fatty acids in green coffee (Coffea arabica L.) beans and chemometric modeling for tracing geographical origins from Ethiopia. Journal of the Science of Food and Agriculture, 99(8): 3811–3823, DOI: https://doi.org/10.1002/jsfa.9603.
Pereira, A. G., Jiménez-López, C., Corral, M. F., Lopes, C., and Simal-Gandara, J., 2020. Extraction, properties, and applications of bioactive compounds obtained from microalgae. Current Pharmaceutical Design, 26: 1929–1950, DOI: https://doi.org/10.2174/1381612826666200403172206.
Ratnayake, W. M. N., Olsson, B., Matthews, D., and Ackman, R. G, 1988. Preparation of omega-3 PUFA concentrates from fish oils via urea complexation. Fett/Lipid, 90(10): 381–386, DOI: https://doi.org/10.1002/lipi.19880901002.
Sharma, N. K., and Rai, A. K., 2011. Biodiversity and biogeography of microalgae: Progress and pitfalls. Environmental Reviews, 19: 1–15, DOI: https://doi.org/10.1139/a10-020.
Vazquez, L., Prados, I., Reglero, G., and Torres, C. F., 2017. Identification and quantification of ethyl carbamate occurring in urea complexation processes commonly utilized for polyun-saturated fatty acid concentration. Food Chemistry, 229: 28–34, DOI: https://doi.org/10.1016/j.foodchem.2017.01.123.
Wang, F. F., Gao, B. Y., Dai, C. M., Su, M., and Zhang, C. W., 2020. Comprehensive utilization of the filamentous oleaginous microalga Tribonema utriculosum for the production of lipids and chrysolaminarin in a biorefinery concept. Algal Research, 50: 101973, DOI: https://doi.org/10.1016/j.algal.2020.101973.
Wang, F. F., Gao, B. Y., Huang, L. D., Su, M., Dai, C. M., and Zhang, C. W., 2018. Evaluation of oleaginous eustigmatophycean microalgae as potential biorefinery feedstock for the production of palmitoleic acid and biodiesel. Bioresource Technology, 270: 30–37, DOI: https://doi.org/10.1016/j.biortech.2018.09.016.
Wille, J. J., and Kydonieus, A., 2003. Palmitoleic acid isomer (C16:1A6) in human skin sebum is effective against gram-positive bacteria. Skin Pharmacology & Applied Skin Physiology, 16(3): 176–87, DOI: https://doi.org/10.1159/000069757.
Xu, Z. J., Hu, Q., Liu, G. X., Xu, Z. J., Zhang, T., and Zhang, C. W., 2018. Novel lipid accumulation pattern in a filamentous oleaginous microalga Tribonema utriculosum SAG22.94 under different initital nitrogen concentrations. Plant Science Journal, 36: 411–419 (in Chinese with English abstract).
Zhang, W. Y., Wang, F. F., Gao, B. Y., Huang, L. D., and Zhang, C. W., 2018a. An integrated biorefinery process: Stepwise extraction of fucoxanthin, eicosapentaenoic acid and chrysolaminarin from the same Phaeodactylum tricornutum biomass. Algal Research, 32: 193–200, DOI: https://doi.org/10.1016/j.algal.2018.04.002.
Zhang, Y., Feng, R., Li, L., Xun, Z., Li, Z., Jia, R., et al., 2018b. The antibacterial mechanism of terpinen-4-ol against Streptococcus agalactiae. Current Microbiology, 75: 1214–1220, DOI: https://doi.org/10.1007/s00284-018-1512-2.
Zhao, W., Fang, H. H., Gao, B. Y., Dai, C. M., Liu, Z. Z., Zhang, C. W., et al., 2020. Dietary Tribonema sp. supplementation increased growth performance, antioxidant capacity, immunity and improved hepatic health in golden pompano (Trachinotus ovatus). Aquaculture, 529: 735667, DOI: https://doi.org/10.1016/j.aquaculture.2020.735667.
Zhou, W. J., Wang, H., Chen, L., Cheng, W. T., and Liu, T. X., 2017. Heterotrophy of filamentous oleaginous microalgae Tribonema minus for potential production of lipid and palmitoleic acid. Bioresource Technology, 239: 250–257, DOI: https://doi.org/10.1016/j.biortech.2017.05.045.
Acknowledgements
This work was supported by the Jiangsu Provincial Natural Science Foundation (No. BK20200734), the Hubei Provincial Natural Science Foundation (No. 2021CFB224), the Engineering Research Center for Tropical and Subtropical Aquatic Ecological Engineering, Ministry of Education, Jinan University, P. R. China (No. 2021A0401), the Research and Innovation Initiatives of Wuhan Polytechnic University (WHPU) (No. 2021Y06), the Hubei Key Laboratory for Processing and Transformation of Agricultural Products (WHPU, China) (No. 2020HBSQGDKFB17), and the Sinopec Joint Program of China Petroleum and Chemical Corporation (No. ST18005-2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, F., Guo, Y., Cao, Y. et al. In vitro Antibacterial Activity of Palmitoleic Acid Isolated from Filamentous Microalga Tribonema minus Against Fish Pathogen Streptococcus agalactiae. J. Ocean Univ. China 21, 1615–1621 (2022). https://doi.org/10.1007/s11802-022-5047-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-022-5047-6