Abstract
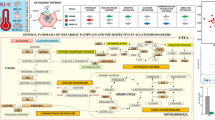
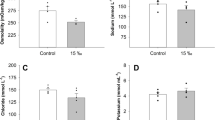
As a stenohaline species, the survival of Sepia pharaonis can be affected by salinity significantly. This study aimed to explore the function of decreasing salinity on the survival of Sepia pharaonis, which can provide an advanced production guide on the culture of S. pharaonis in the rainy season. Salinity was gradually decreased from 29 to 22 within 48 h to acclimate S. pharaonis to a low-salinity environment. After ten days of breeding under low-salinity of 22, the death rate was high. In this process, changes in tissue and cell structures in the larval liver, biochemical indicators, and osmoregulation-related gene expression were examined. Interestingly, hepatocytes in the low-salinity group were irregular, had dissolved tissue inclusions, and contained vacuolized cells. Therefore, low salinity caused severe damages at a cellular level that can elevate the mortality rate. A gradual decline in salinity limited the full adaptation of S. pharaonis. Biochemical indicators and osmoregulation-related gene expression changed similarly. For instance, the trend of malondialdehyde (MAD) as a product of lipid peroxidation reflected the degree of damage to the body by free radicals. The antioxidant system of S. pharaonis could cope with oxidative stress caused by the change in salinity to a certain extent. Osmoregulation-related genes’ expression also showed an optimistic result, that is, S. pharaonis responded positively to the change in salinity by adjusting the expression of osmoregulation-related genes. Conversely, the increase in mortality at day 10 also proved the weak adaptation capability of S. pharaonis. This study indicated that S. pharaonis can adapt to a low-salinity environment with a limited extent.
Similar content being viewed by others
References
Bystriansky, J. S., and Schulte, P. M., 2011. Changes in gill H+-ATPase and Na+/K+-ATPase expression and activity during freshwater acclimation of Atlantic salmon (Salmo salar). Journal of Experimental Biology, 214(14): 2435, https://doi.org/10.1242/jeb.050633.
Cutler, C. P., Sanders, I. L., Hazon, N., and Cramb, G., 1995. Primary sequence, tissue specificity and expression of the Na+, K+-ATPase α 1 subunit in the European eel (Anguilla anguilla). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 111(4): 567–573, https://doi.org/10.1016/0305-0491(95)00037-9.
Deane, E. E., and Woo, N. Y., 2005. Cloning and characterization of sea bream Na+-K+-ATPase α and β subunit genes: In vitro effects of hormones on transcriptional and translational expression. Biochemical and Biophysical Research Communications, 331(4): 1229–1238, https://doi.org/10.1016/j.bbrc.2005.04.038.
Deane, E. E., and Woo, N. Y., 2006. Tissue distribution, effects of salinity acclimation, and ontogeny of aquaporin 3 in the marine teleost, silver sea bream (Sparus sarba). Marine Biotechnology, 8(6): 663–671, https://doi.org/10.1007/s10126-006-6001-0.
DeFaveri, J., and Merilä, J., 2014. Local adaptation to salinity in the three-spined stickleback?. Journal of Evolutionary Biology, 27(2): 290–302, https://doi.org/10.1111/jeb.12289.
Han, X., Liu, P., Gao, B., Wang, H., Duan, Y., Xu, W., et al., 2015. Na+/K+-ATPase α-subunit in swimming crab Portunus trituberculatus: Molecular cloning, characterization, and expression under low salinity stress. Chinese Journal of Oceanology and Limnology, 33(004): 828–837, https://doi.org/10.1007/s00343-015-4018-9.
He, S., Shelly, D. A., Moseley, A. E., James, P. F., James, J. H., Paul, R. J., et al., 2001. The α1- and α2-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 281(3): R917–R925, https://doi.org/10.1152/ajpregu.2001.281.3.R917.
Heckwolf, M. J., Meyer, B. S., Döring, T., Eizaguirre, C., and Reusch, T. B., 2018. Transgenerational plasticity and selection shape the adaptive potential of sticklebacks to salinity change. Evolutionary Applications, 11(10): 1873–1885, https://doi.org/10.1111/eva.12688.
Hendrix Jr., J., Hulet, W., and Greenberg, M., 1981. Salinity tolerance and the responses to hypoosmotic stress of the bay squid Lolliguncula brevis, a euryhaline cephalopod mollusc. Comparative Biochemistry and Physiology Part A: Physiology, 69(4): 641–648, https://doi.org/10.1016/0300-9629(81)90150-X.
Hwang, P., Fang, M., Tsai, J. C., Huang, C., and Chen, S., 1998. Expression of mRNA and protein of Na+-K+-ATPase α sub-unit in gills of tilapia (Oreochromis mossambicus). Fish Physiology and Biochemistry, 18(4): 363–373, https://doi.org/10.1023/A:1007711606064.
Jaffer, Y. D., Saraswathy, R., Ishfaq, M., Antony, J., and Sharma, P. C., 2019. Effect of low salinity on the growth and survival of juvenile Pacific white shrimp, Penaeus vannamei: A revival. Aquaculture, 515: 734561, https://doi.org/10.1016/j.aquaculture.2019.734561.
James, P. F., Grupp, I. L., Grupp, G., Woo, A. L., Askew, G. R., Croyle, M. L., et al., 1999. Identification of a specific role for the Na, K-ATPase α2 isoform as a regulator of calcium in the heart. Molecular Cell, 3(5): 555–563, https://doi.org/10.1016/S1097-2765(00)80349-4.
Leeuwenburgh, C., Hollander, J., Leichtweis, S., Griffiths, M., Gore, M., and Ji, L. L., 1997. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. American Journal of Physiology — Regulatory, Integrative and Comparative Physiology, 272(1): R363–R369, https://doi.org/10.1152/ajpregu.1997.272.1.R363.
Li, E., Wang, X., Chen, K., Xu, C., Qin, J. G., and Chen, L., 2017. Physiological change and nutritional requirement of Pacific white shrimp Litopenaeus vannamei at low salinity. Reviews in Aquaculture, 9(1): 57–75, https://doi.org/10.1111/raq.12104.
MacIver, B., Cutler, C. P., Yin, J., Hill, M. G., Zeidel, M. L., and Hill, W. G., 2009. Expression and functional characterization of four aquaporin water channels from the European eel (Anguilla anguilla). Journal of Experimental Biology, 212(17): 2856–2863, https://doi.org/10.1242/jeb.025882.
Peng, R. B., Wang, P. S., Jiang, M. W., Peng, R., Han, Q. X., and Jiang, X. M., 2016. Effect of salinity on embryonic development of the cuttlefish Sepia pharaonis. Journal of the World Aquaculture Society, 48(4): 666–675, https://doi.org/10.1111/jwas.12321.
Richards, J. G., Semple, J. W., Bystriansky, J. S., and Schulte, P. M., 2003. Na+/K+-ATPase α-isoform switching in gills of rainbow trout (Oncorhynchus mykiss) during salinity transfer. Journal of Experimental Biology, 206(24): 4475–4486, https://doi.org/10.1242/jeb.00701.
Sun, M., Jiang, K., Zhang, F., Zhang, D., Shen, A., Jiang, M., et al., 2012. Effects of various salinities on Na+-K+-ATPase, Hsp70 and Hsp90 expression profiles in juvenile mitten crabs, Eriocheir sinensis. Genetics and Molecular Research, 11(2): 978–986, https://doi.org/10.4238/2012.April.19.3.
Sun, P., Peng, S. M., Yin, F., and Shi, Z. H., 2010. Effects of salinity on activity of Na+/-K+-ATPase in juvenile Oplegnathus fasciatus. Journal of Fisheries of China, 34(8): 1204–1209.
Tingaud-Sequeira, A., Calusinska, M., Finn, R. N., Chauvigné, F., Lozano, J., and Cerdà, J., 2010. The zebrafish genome encodes the largest vertebrate repertoire of functional aquaporins with dual paralogy and substrate specificities similar to mammals. BMC Evolutionary Biology, 10(1): 38, https://doi.org/10.1186/1471-2148-10-38.
Tipsmark, C. K., Sørensen, K. J., and Madsen, S., 2010. Aquaporin expression dynamics in osmoregulatory tissues of Atlantic salmon during smoltification and seawater acclimation. Journal of Experimental Biology, 213(3): 368–379, https://doi.org/10.1242/jeb.034785.
Vij, S., and Tyagi, A. K., 2008. A20/AN1 zinc-finger domain-containing proteins in plants and animals represent common elements in stress response. Functional & Integrative Genomics, 8(3): 301–307, https://doi.org/10.1007/s10142-008-0078-7.
Wang, R., Huang, X., Wang, H., Lu, J., Shi, X., Feng, G., et al., 2019. Effects of salinity on embryonic and larval development of Chinese mitten crab Eriocheir sinensis (Decapoda: Brachyura) and salinity-induced physiological changes. Journal of Oceanology and Limnology, 37(5): 1777–1788, https://doi.org/10.1007/s00343-019-8190-1.
Xu, C., Li, E., Liu, Y., Wang, X., Qin, J. G., and Chen, L., 2017. Comparative proteome analysis of the hepatopancreas from the Pacific white shrimp Litopenaeus vannamei under long-term low salinity stress. Journal of Proteomics, 162: 1–10, https://doi.org/10.1016/j.jprot.2017.04.013.
Yin, S. J., Zhang, L., Zhang, L., Wan, J., Song, W., Jiang, X., et al., 2018. Metabolic responses and arginine kinase expression of juvenile cuttlefish (Sepia pharaonis) under salinity stress. International Journal of Biological Macromolecules, 113: 881–888, https://doi.org/10.1016/j.ijbiomac.2018.03.036.
Zou, H., He, F., Lan, Z., Tang, L., and Lu, W., 2019. The personality of Japanese flounder (Paralichthys olivaceus) and gene expression related with osmoregulatory capacity in the gills. Aquaculture, 500: 221–227, https://doi.org/10.1016/j.aquaculture.2018.10.013.
Acknowledgements
This study was supported by the Ningbo Agricultural Major Projects (No. 201401C1111001) and the Foundation of Zhejiang Educational Committee (No. Y2019409 57), and all the authors were sponsored by K. C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xin, H., Wu, K., Yuan, Y. et al. Physiological and Molecular Analyses of Low-Salinity Stress Response in the Cuttlefish (Sepia pharaonis) Juveniles. J. Ocean Univ. China 21, 969–976 (2022). https://doi.org/10.1007/s11802-022-4880-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-022-4880-y