Abstract
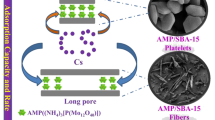
Short channel platelet SBA-15 mesoporous material is one of the effective adsorbent for the recovery of U(VI) from aqueous solution. Nevertheless, the defect is that the mesoporous size limits the total stripping of attached U(VI) in recycling use and the increasing densities of organic groups after functionalization. Thus, a simple and controllable method was adopted to prepare SBA-15-type material with expanded pore channel by adding trimethylbenzene in synthesis procedure. The structure, morphology and functional groups were characterized by scanning electron microscope, powder X-ray diffraction, thermogravimetric analysis, transmission electron microscope, FTIR and N2 adsorption-desorption isotherm analysis. Furthermore, the adsorption behavior of obtained product was test under various factors such as initial concentration, pH, elution rate and contact time. The pore expanded platelet SBA-15 exhibited higher U(VI) adsorption capacity, higher elution rate, and more bearing of amidoxime groups. Due to the higher amidoxime groups, the adsorption capacity of U(VI) on the amidoxime functionalized pore-expanded SBA-15 was 674mg-U g−1. The results show that the simple and controllable pore-expanded method is an effective strategy to enhance the elution effect and increase grafting amount of organic groups on the mesoporous materials.
Similar content being viewed by others
References
Bayramoglu, G., and Arica, M. Y., 2016. MCM-41 silica particles grafted with polyacrylonitrile: Modification in to amidoxime and carboxyl groups for enhanced uranium removal from aqueous medium. Microporous and Mesoporous Materials, 226: 117–124.
Benamor, T., Michelin, L., Lebeau, B., and Marichal, C., 2012. Flash induction calcination: A powerful tool for total template removal and fine tuning of the hydrophobic/hydrophilic balance in SBA-15 type silica mesoporous materials. Microporous and Mesoporous Materials, 147: 334–342.
Bryant, D. E., Stewart, D. I., Kee, T. P., and Barton, C. S., 2003. Development of a functionalized polymer-coated silica for the removal of uranium from groundwater. Environmental Science & Technology, 37: 4011–4016.
Chen, S. Y., Chen, Y. T., Lee, J. J., and Cheng, S., 2011. Tuning pore diameter of platelet SBA-15 materials with short mesochannels for enzyme adsorption. Journal of Materials Chemistry, 21: 5693–5703.
Cheng, Y. H., Zhou, L. B., Xu, J. X., Miao, C. X., Hua, W. M., Yue, Y. H., and Gao, Z., 2016. Chromium-based catalysts for ethane dehydrogenation: Effect of SBA-15 support. Microporous and Mesoporous Materials, 234: 370–376.
Dai, Y., Jin, J., Zhou, L., Li, T., Li, Z., Liu, Z., Huang, G., and Adesina, A. A., 2017. Preparation of hollow SiO2 microspheres functionalized with amidoxime groups for highly efficient adsorption of U(VI) from aqueous solution. Journal of Radioanalytical and Nuclear Chemistry, 311: 2029–2037.
Fasfous, I. I., and Dawoud, J. N., 2012. Uranium(VI) sorption by multiwalled carbon nanotubes from aqueous solution. Applied Surface Science, 259: 433–440.
Feuston, B. P., and Higgins, J. B., 1994. Model structures for MCM-41 materials: A molecular dynamics simulation. Journal of Physical Chemistry, 98: 4459–4462.
Goldberg, S., 2005. Inconsistency in the triple layer model description of ionic strength dependent boron adsorption. Journal of Colloid and Interface Science, 285 (2): 509–517.
Guo, W., Chen, R., Liu, Y., Meng, M., Meng, X., Hu, Z., and Song, Z., 2013. Preparation of ion-imprinted mesoporous silica SBA-15 functionalized with triglycine for selective adsorption of Co(II). Colloid Surface A: Physicochemical and Engineering Aspects, 436: 693–703.
Jang, J. H., Dempsey, B. A., and Burgos, W. D., 2007. A model-based evaluation of sorptive reactivities of hydrous ferric oxide and hematite for U(VI). Environmental Science & Technology, 41: 4305–4310.
Ji, G. J., Zhu, G. R., Wang, X. H., Wei, Y. L., Yuan, J. S., and Gao, C. J., 2017. Preparation of amidoxime functionalized SBA-15 with platelet shape and adsorption property of U(VI). Separation and Purification Technology, 174: 455–465.
Kamari, A., Ngah, W. S. W., Chong, M. Y., and Cheah, M. L., 2009. Sorption of acid dyes onto GLA and H2SO4 cross-linked chitosan beads. Desalination, 249: 1180–1189.
Kango, S., Kalia, S., Celli, A., Njuguna, J., Habibi, Y., and Kumar, R., 2013. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites-A review. Progress in Polymer Science, 38: 1232–1261.
Kowal-Fouchard, A., Drot, R., Simoni, E., and Ehrhardt, J. J., 2004. Use of spectroscopic techniques for Uranium(VI)/Montmorillonite interaction modeling. Environmental Science & Technology, 38: 1399–1407.
Li, X., Ding, C., Liao, J., Lan, T., Li, F., Zhang, D., Yang, J., Yang, Y., Luo, S., Tang, J., and Liu, N., 2014. Biosorption of uranium on Bacillus sp. dwc-2: Preliminary investigation on mechanism. Journal of Environmental Radioactivity, 135: 6–12.
Lützenkirchen, J., 1997. Ionic strength effects on cation sorption to oxides: Macroscopic observations and their significance in microscopic interpretation. Journal of Colloid and Interface Science, 195 (1): 149–155.
Mellah, A., Chegrouche, S., and Barkat, M., 2006. The removal of uranium(VI) from aqueous solutions onto activated carbon: Kinetic and thermodynamic investigations. Journal of Colloid and Interface Science, 296: 434–441.
Ojeda-López, R., Pérez-Hermosillo, I. J., Esparza-Schulz, J. M., Cervantes-Uribe, A., and Domínguez-Ortiz, A., 2015. SBA-15 materials: Calcination temperature influence on textural properties and total silanol ratio. Adsorption, 21: 659–669.
Pang, H. W., Wu, Y. H., Wang, X. X., Hu, B. W., and Wang, X. K., 2019. Recent advances in composites of graphene and layered double hydroxides for water remediation: A review. Chemistry-An Asian Journal, 14: 2542–2552.
Rahmati, A., Ghaemi, A., and Samadfam, M., 2012. Kinetic and thermodynamic studies of uranium (VI) adsorption using Amberlite IRA-910 resin. Annals of Nuclear Energy, 39: 42–48.
Ren, Y. M., Yang, R. Z., Shao, L., Tang, H., Wang, S. F., Zhao, J. L., Zhong, J. R., and Kong, C. P., 2016. The removal of aqueous uranium by SBA-15 modified with phosphoramide: A combined experimental and DFT study. RSC Advances, 6: 68695–68704.
Schindler, M., Hawthorne, F. C., Freund, M. S., and Burns, P. C., 2009. XPS spectra of uranyl minerals and synthetic uranyl compounds. I: The U 4f spectrum. Geochimica et Cosmochimica Acta, 73: 2471–2487.
Sholl, D. S., and Lively, R. P., 2016. Seven chemical separations to change the world. Nature, 532: 435–437.
Sujandi, P. S. E., Han, D. S., Han, S. C., Jin, M. J., and Ohsuna, T., 2006. Amino-functionalized SBA-15 type mesoporous silica having nanostructured hexagonal platelet morphology. Chemical Communications, 27: 4131–4133.
Wang, L. F., and Yang, R. T., 2011. Increasing selective CO2adsorption on amine-grafted SBA-15 by increasing silanol density. Journal of Physical Chemistry C, 115: 21264–21272.
Wang, X. H., Zhu, G. R., and Guo, F., 2013. Removal of uranium(VI) ion from aqueous solution by SBA-15. Annals of Nuclear Energy, 56: 151–157.
Wang, X. J., Ji, G. J., Zhu, G. R., Song, C. H., Zhang, H., and Gao, C. J., 2019. Surface hydroxylation of SBA-15 via alkaline for efficient amidoxime-functionalization and enhanced uranium adsorption. Separation and Purification Technology, 209: 623–635.
Wang, X. X., Chen, L., Wang, L., Fan, Q. H., Pan, D. Q., Li, J. X., Chi, F. T., Xie, Y., Yu, S. J., Xiao, C. L., Luo, F., Wang, J., Wang, X. L., Chen, C. L., Wu, W. S., Shi, W. Q., Wang, S. A., and Wang, X. K., 2019. Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Science China Chemistry, 62: 933–967.
Wang, Y., Gu, Z., Yang, J., Liao, J., Yang, Y., Liu, N., and Tang, J., 2014. Amidoxime-grafted multiwalled carbon nanotubes by plasma techniques for efficient removal of uranium(VI). Applied Surface Science, 320: 10–20.
Wang, Y. L., Song, L. J., Zhu, L., Guo, B. L., Chen, S. W., and Wu, W. S., 2014. Removal of uranium(VI) from aqueous solution using iminodiacetic acid derivative functionalized SBA-15 as adsorbents. Dalton Transactions, 43: 3739–3749.
Wang, Y., Noguchi, M., Takahashi, Y., and Ohtsuka, Y., 2001. Synthesis of SBA-15 with different pore sizesand the utilization as supports of high loading of cobalt catalysts. Catalysis Today, 68: 3–9.
Webb, S. M., Fuller, C. C., Tebo, B. M., and Bargar, J. R., 2006. Determination of Uranyl incorporation into biogenic Manganese oxides using X-ray absorption spectroscopy and scattering. Environmental Science & Technology, 40: 771–777.
Xing, S. Y., Lv, P. M., Fu, J. Y., Wang, J. Y., Fan, P., Yang, L. M., and Yuan, Z. H., 2017. Direct synthesis and characterization of pore-broadened Al-SBA-15. Microporous and Mesoporous Materials, 239: 316–327.
Yang, L., Lei, W., Bo, L., Zhang, M., Rui, W., Guo, X., Xing, L., Ji, Z., Li, S., and Ma, L., 2016. Pore-free matrix with cooperative chelating of hyperbranched ligands for high-performance separation of uranium. ACS Applied Materials & Interfaces, 8: 28853–28861.
Yang, L. M., Wang, Y. J., Luo, G. S., and Dai, Y. Y., 2005. Functionalization of SBA-15 mesoporous silica with thiol or sulfonic acid groups under the crystallization conditions. Microporous and Mesoporous Materials, 84: 275–282.
Yang, P., Chen, R., Liu, Q., Zhang, H., Liu, J., Yu, J., Liu, P., Bai, X., and Wang, J., 2019. The efficient immobilization of uranium(VI) by modified dendritic fibrous nanosilica (DFNS) using mussel bioglue. Inorganic Chemistry Frontiers, 6: 746–755.
Yang, P. P., Liu, Q., Liu, J. Y., Chen, R. R., Li, R. M., Bai, X. F., and Wang, J., 2019. Highly efficient immobilization of uranium(VI) from aqueous solution by phosphonate-functionalized dendritic fibrous nanosilica (DFNS). Journal of HazardousMaterials, 363: 248–257.
Yang, S. T., Zong, P. F., Ren, X. M., Wang, Q., and Wang, X. K., 2012. Rapid and highly efficient preconcentration of Eu(III) by core-shell structured Fe3O4@humic acid magnetic nanoparticles. ACS Applied Materials & Interfaces, 4 (12): 6891–6900.
Yang, S. Y., Li, Q., Chen, L., Chen, Z. S., Hu, B. W., Wang, H. H., and Wang, X. K., 2020. Synergistic removal and reduction of U(VI) and Cr(VI) by Fe3S4 micro-crystal. Chemical Engineering Journal, 385: 123909.
Yin, L., Hu, B. W., Zhuang, L., Fu, D., Li, J., Hayat, T., Alsaedi, A., and Wang, X. K., 2020. Synthesis of flexible cross-linked cryptomelane-type manganese oxide nanowire membranes and their application for U(VI) and Eu(III) elimination from solutions. Chemical Engineering Journal, 381: 122744.
Yuan, L. Y., Liu, Y. L., Shi, W. Q., Li, Z. J., Lan, J. H., Feng, Y. X., Zhao, Y. L., Yuan, Y. L., and Chai, Z. F., 2012. A novel mesoporous material for uranium extraction, dihydroimidazole functionalized SBA-15. Journal of Materials Chemistry, 22: 17019–17026.
Zhang, A., Asakura, T., and Uchiyama, G., 2003. The adsorption mechanism of uranium(VI) from seawater on a macroporous fibrous polymeric adsorbent containing amidoxime chelating functional group. Reactive & Functional Polymers, 57: 67–76.
Zhao, Y. G., Li, J. X., Zhang, S. W., and Wang, X. K., 2014. Amidoxime-functionalized magnetic mesoporous silica for selective sorption of U(VI). RSC Advances, 4: 32710–32717.
Zhao, Y. G., Li, J. X., Zhao, L. P., Zhang, S. W., Huang, Y. S., Wu, X. L., and Wang, X. K., 2014. Synthesis of amidoxime-functionalized Fe3O4@SiO2 core-shell magnetic microspheres for highly efficient sorption of U(VI). Chemical Engineering Journal, 235: 275–283.
Zhao, Y. G., Wang, X. X., Li, J. X., and Wang, X. K., 2015. Amidoxime functionalization of mesoporous silica and its high removal of U(VI). Polymer Chemistry, 6: 5376–5384.
Zhe, W., Chao, X., Lu, Y., Wu, F., Gang, Y., Wei, G., Sun, T., and Jing, C., 2017. Visualization of adsorption: Luminescent mesoporous silica-carbon dots composite for rapid and selective removal of U(VI) and in-situ monitoring the adsorption behavior. ACS Applied Materials & Interfaces, 9: 7392–7398.
Zheng, H., Zhou, L. M., Liu, Z. R., Le, Z. G., Ouyang, J. B., Huang, G. L., and Shehzad, H., 2019. Functionalization of mesoporous Fe3O4@SiO2 nanospheres for highly efficient U(VI) adsorption. Microporous and Mesoporous Materials, 279: 316–322.
Zienkiewicz-Strzalka, M., Derylo-Marczewska, A., and Kozakevych, R. B., 2018. Silica nanocomposites based on silver nanoparticles-functionalization and pH effect. Applied Nanoscience, 8: 1649–1668.
Zou, H., Zhou, L., Huang, Z., Liu, Z., and Luo, T., 2017. Characteristics of equilibrium and kinetic for U(VI) adsorption using novel diamine-functionalized hollow silica microspheres. Journal of Radioanalytical and Nuclear Chemistry, 311: 269–278.
Acknowledgements
This work is financially supported by the Fundamental Research Funds for the Central Universities (No. 201964 020), and the National Natural Science Foundation of China (No. U1607124).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ji, G., Wang, X., Zhu, G. et al. Facile Route to Synthesize Pore Expanded Platelet SBA-15 for Enhanced Amidoxime-Functionalization and Efficient Extraction of U(VI) from Aqueous Solution. J. Ocean Univ. China 19, 1103–1115 (2020). https://doi.org/10.1007/s11802-020-4424-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-020-4424-2