Abstract
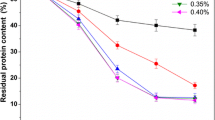
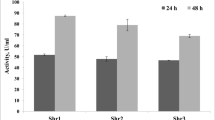
The industrial processing of shrimp produces massive quantities of solid waste that is a notable source of animal protein, chitin, carotenoids, and other bioactive compounds that are not appropriately utilized. In the present study, chitin and protein extraction from shrimp head with autolysis and fermentation using Bacillus licheniformis were investigated. The results showed that when shrimp heads were autolyzed with a natural pH at 50°C for 4 h, the total amino acid nitrogen in the supernatant was 5.01 mg mL−1. Then, when a 50% (v/m) inoculum of the hydrolysate was incubated at 60°C for 10 h, a deproteinization rate of 88.3% could be obtained. The fermented supernatant was processed into a dry protein powder, while the residues were demineralized by 10% citric acid for chitin. The recovered protein powder contained 5.5% moisture, 11.5% ash, and 66.7% protein, while the chitin contained 3.5% moisture, 2.1% ash, and 3.1% protein. In addition, amino acids, minerals, heavy metals, the degree of acetylation, microstructure, and Fourier-transform infrared (FT-IR) spectroscopy results were analyzed. Furthermore, the statistics of the large scale trial after treatment with 20 kg of shrimp heads were analyzed. Thus, this work made the shrimp waste utilization environmentally sound and valuable.
Similar content being viewed by others
References
Altundag, H., and Tuzen, M., 2011. Comparison of dry, wet and microwave digestion methods for the multi element determination in some dried fruit samples by ICP-OES. Food and Chemical Toxicology, 49 (11): 2800–2807.
Bueno-Solano, C., López-Cervantes, J., Campas-Baypoli, O. N., Lauterio-García, R., Adan-Bante, N. P., and Sánchez-Machado, D. I., 2009. Chemical and biological characteristics of protein hydrolysates from fermented shrimp by-products. Food Chemistry, 112 (3): 671–675.
Cao, W., Zhang, C., Hong, P., and Ji, H., 2008. Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chemistry, 109 (1): 176–183.
Cao, W. H., Zhang, C. H., Hong, P. Z., Ji, H. W., Hao, J. M., and Zhang, J., 2009. Autolysis of shrimp head by gradual temperature and nutritional quality of the resulting hydrolysate. LWT-Food Science and Technology, 42 (1): 244–249.
Cuniff, P., 1997. Official Methods of Analysis of AOAC International, Chapter 7. Arlington, Virginia, 46–48.
Czechowska-Biskup, R., Jarosińska, D., Rokita, B., Ulański, P., and Rosiak, J. M., 2012. Determination of degree of deacetylation of chitosan-comparision of methods. Progress on Chemistry and Application of Chitin and Its Derivatives, 17: 5–20.
Ellingson, D., Pritchard, T., Foy, P., King, K., Mitchell, B., Austad, J., Winters, D., and Sullivan, D., 2012. Analysis of free and total myo-inositol in foods, feeds, and infant formula by high-performance anion exchange chromatography with pulsed amperometric detection, including a novel total extraction using microwave-assisted acid hydrolysis and enzymatic treatment. Journal of AOAC International, 95 (5): 1469–1478.
FAO, R. F., 2014. The State of World Fisheries and Aquaculture 2014. Opportunities and Challenges. Food and Agriculture Organization of the U. N.
FAO/WHO/UNU, 2007. Protein and amino acid requirements in human nutrition. World Health Organization Technical Report, Series 935.
Gortari, M. C., and Hours, R. A., 2013. Biotechnological processes for chitin recovery out of crustacean waste: A minireview. Electronic Journal of Biotechnology, 16 (13): 1–18.
Hajji, S., Younes, I., Ghorbel-Bellaaj, O., Hajji, R., Rinaudo, M., Nasri, M., and Jellouli, K., 2014. Structural differences between chitin and chitosan extracted from three different marine sources. International Journal of Biological Macromolecules, 65 (5): 298–306.
Herwig, N., Stephan, K., Panne, U., Pritzkow, W., and Vogl, J., 2011. Multi-element screening in milk and feed by SF-ICPMS. Food Chemistry, 124 (3): 1223–1230.
Kaya, M., Baran, T., and Karaarslan, M., 2015. A new method for fast chitin extraction from shells of crab, crayfish and shrimp. Natural Product Research, 29 (15): 1477–1480.
Kaya, M., Dudakli, F., Asan-Ozusaglam, M., Cakmak, Y. S., Baran, T., Mentes, A., and Erdogan, S., 2016. Porous and nanofiber α-chitosan obtained from blue crab (Callinectes sapidus) tested for antimicrobial and antioxidant activities. LWT-Food Science and Technology, 65: 1109–1117.
Knorr, D., 1991. Recovery and utilization of chitin and chitosan in food processing waste management. Food Technology, 45: 114–122.
Kurita, K., Tomita, K., Tada, T., Ishii, S., Nishimura, S. I., and Shimoda, K., 1993. Squid chitin as a potential alternative chitin source: Deacetylation behavior and characteristic properties. Journal of Polymer Science Part A: Polymer Chemistry, 31 (2): 485–491.
Lavall, R. L., Assis, O. B. G., and Campana-Filho, S. P., 2007. β-Chitin from the pens of Loligo sp.: Extraction and characterization. Bioresource Technology, 98 (13): 2465–2472.
Liu, P., Liu, S., Guo, N., Mao, X., Lin, H., Xue, C., and Wei, D., 2014. Cofermentation of Bacillus licheniformis and Gluconobacter oxydans for chitin extraction from shrimp waste. Biochemical Engineering Journal, 91: 10–15.
Lopes, C., Antelo, L. T., Franco-Uría, A., Alonso, A. A., and Pérez-Martín, R., 2017. Chitin production from crustacean biomass: Sustainability assessment of chemical and enzymatic processes. Journal of Cleaner Production, 172: 4140–4151.
Mahmoud, N. S., Ghaly, A. E., and Arab, F., 2007. Unconventional approach for demineralization of deproteinized crustacean shells for chitin production. American Journal of Biochemistry & Biotechnology, 3 (1): 1–9.
Mao, X., Guo, N., Sun, J., and Xue, C., 2017. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. Journal of Cleaner Production, 143: 814–823.
Mao, X., Pei, L., Shuai, H., Xie, J., Kan, F., Yu, C., Li, Z., Xue, C., and Hong, L., 2013a. Antioxidant properties of bio-active substances from shrimp head fermented by Bacillus licheniformis OPL-007. Applied Biochemistry and Biotechnology, 171 (5): 1240–1252.
Mao, X., Zhang, J., Kan, F., Gao, Y., Lan, J., Zhang, X., Hu, Z., Li, Y., and Lin, H., 2013b. Antioxidant production and chitin recovery from shrimp head fermentation with Streptococcus thermophilus. Food Science and Biotechnology, 22 (4): 1023–1032.
Mizani, M., Aminlari, M., and Khodabandeh, M., 2005. An effective method for producing a nutritive protein extract powder from shrimp-head waste. Food Science and Technology International, 11 (1): 49–54.
Percot, A., Viton, C., and Domard, A., 2003. Optimization of chitin extraction from shrimp shells. Biomacromolecules, 4 (1): 12–18.
Poothawan, T., and Lomthaisong, K., 2015. Analysis of chitin, chitosan, and optimization for carotenoids extraction yield with rice bran oil from Thai fairy shrimp. Chiang Mai Journal of Science, 42 (4): 918–929.
Rao, M. S., and Stevens, W. F., 2005. Chitin production by Lactobacillus fermentation of shrimp biowaste in a drum reactor and its chemical conversion to chitosan. Journal of Chemical Technology and Biotechnology, 80 (9): 1080–1087.
Sachindra, N. M., and Mahendrakar, N. S., 2005. Process optimization for extraction of carotenoids from shrimp waste with vegetable oils. Bioresource Technology, 96 (10): 1195–1200.
Sánchez-Machado, D. I., López-Cervantes, J., López-Hernández, J., Paseiro-Losada, P., and Simal-Lozano, J., 2003. High-per formance liquid chromatographic analysis of amino acids in edible seaweeds after derivatization with phenyl isothiocyanate. Chromatographia, 58 (3): 159–163.
Senphan, T., Benjakul, S., and Kishimura, H., 2014. Characteristics and antioxidative activity of carotenoprotein from shells of Pacific white shrimp extracted using hepatopancreas proteases. Food Bioscience, 5: 54–63.
Shahidi, F., and Brown, J. A., 1998. Carotenoid pigments in seafoods and aquaculture. Critical Reviews in Food Science and Nutrition, 38 (1): 1–67.
Silva, C. P. D., Bezerra, R. S., Santos, A. C. O. D., Messias, J. B., Castro, C. R. O. B. D., and Junior, L. B. C., 2017. Biological value of shrimp protein hydrolysate by-product produced by autolysis. LWT-Food Science and Technology, 80: 456–461.
Son, H. Y., Kim, H., and Kwon, Y. H., 2007. Taurine prevents oxidative damage of high glucose-induced cataractogenesis in isolated rat lenses. Journal of Nutritional Science and Vitaminology, 53 (4): 324–330.
Sowmya, R., Rathinaraj, K., and Sachindra, N. M., 2011. An autolytic process for recovery of antioxidant activity rich carotenoprotein from shrimp heads. Marine Biotechnology, 13 (5): 918–927.
Sriket, P., Benjakul, S., Visessanguan, W., and Kijroongrojana, K., 2007. Comparative studies on chemical composition and thermal properties of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) meats. Food Chemistry, 103 (4): 1199–1207.
Sun, J., and Mao, X., 2016. An environmental friendly process for Antarctic krill (Euphausia superba) utilization using fermentation technology. Journal of Cleaner Production, 127: 618–623.
Synowiecki, J., and Al Khateeb, N. A. A. Q., 2000. The recovery of protein hydrolysate during enzymatic isolation of chitin from shrimp Crangon crangon processing discards. Food Chemistry, 68 (2): 147–152.
Tesanovic, D., Kalenjuk, B., Tesanovic, D., Psodorov, D., Ristic, Z., and Markovic, V., 2011. Changes of biochemical and sensory characteristics in the musculus longissimus dorsi of the fallow deer in the early phase post-mortem and during maturation. African Journal of Biotechnology, 10 (55): 11668–11675.
Verhaeghe, T., Vlaemynck, G., Block, J. D., Weyenberg, S. V., and Hendrickx, M., 2016. Thermal inactivation kinetics of proteases and polyphenoloxidase in brown shrimp (Crangon crangon). Food Chemistry, 197: 641–647.
Yang, J. K., Shih, I. L., Tzeng, Y. M., and Wang, S. L., 2000. Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes. Enzyme and Microbial Technology, 26 (5): 406–413.
Yu, Z., and Lau, D., 2017. Flexibility of backbone fibrils in α-chitin crystals with different degree of acetylation. Carbohydrate Polymers, 174 (15): 941–947.
Zhang, H. C., Jin, Y. F., Deng, Y., Wang, D. F., and Zhao, Y. Y., 2012. Production of chitin from shrimp shell powders using Serratia marcescens B742 and Lactobacillus plantarum ATCC 8014 successive two-step fermentation. Carbohydrate Research, 362 (2): 13–20.
Acknowledgments
This work was supported by China Agriculture Research System (No. CARS-48), the Major Special Science and Technology Projects in Shandong Province (No. 2016 YYSP016), the Ningbo Science and Technology Projects (No. 2017C110006), the Shandong Provincial Natural Science Foundation, China (No. ZR2015CQ021) and the Fundamental Research Funds for the Central Universities (No. 201564018).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Guo, N., Sun, J., Zhang, Z. et al. Recovery of Chitin and Protein from Shrimp Head Waste by Endogenous Enzyme Autolysis and Fermentation. J. Ocean Univ. China 18, 719–726 (2019). https://doi.org/10.1007/s11802-019-3867-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-019-3867-9