Abstract
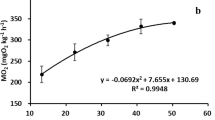
This study was conducted to evaluate the critical thermal maximum (CTMax), the routine metabolism rate (MO2) and the limiting oxygen saturation (LOS) of three salmonids with four different body weights ranging from 16 g to 131 g. The CTMax was estimated at three different heating rates including 0.5°C min−1, 1°C h−1 and 2°C d−1. Results showed that the CTMax of maple trout (Oncorhynchus mykiss) was the highest, which was followed by steelhead trout (O. mykiss) and Atlantic salmon (Salmon salar). The CTMax of the salmonid fish decreased with the increment of body weight, and was significantly influenced by the heating rate. The MO2 of the salmonid fish increased with the increment of temperature, and decreased with the increment of body weight. Suffocation points of the fish decreased with increasing body weight and temperature. Steelhead trout was more tolerant to hypoxia than maple trout and Atlantic salmon, while the MO2 of Atlantic salmon was the highest among these three salmonids. The LOS of the fish generally had a positive trend with temperature and body weight, and the LOS of steelhead trout was significantly lower than that of maple trout and Atlantic salmon. In conclusion, maple trout was the most tolerant kind to high temperature, while steelhead trout was the most tolerant to hypoxia among three kinds of salmonids. Moreover, the abilities to tolerate higher temperature of three salmonids were affected by their body weight and the heating rate, while the abilities to tolerate hypoxia were influenced by their body weight and the water temperature.
Similar content being viewed by others
References
Atkins, M. E., and Benfey, T. J., 2008. Effect of acclimation temperature on routine metabolic rate in triploid salmonids. Comparative Biochemistry and Physiology Part A Molecular and Integrative Physiology, 149 (2): 157–161.
Baker, S. C., and Heidinger, R. C., 1996. Upper lethal temperature tolerance of fingerling black crappie. Journal of Fish Biology, 48 (6): 1123–1129.
Barnes, R., King, H., and Carter, C. G., 2011. Hypoxia tolerance and oxygen regulation in Atlantic salmon, Salmo salar from a Tasmanian population. Aquaculture, 318 (3): 397–401.
Becker, C. D., and Genoway, R. G., 1979. Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environmental Biology of Fishes, 4 (3): 245–256.
Beitinger, T. L., Bennett, W. A., and Mccauley, R. W., 2000. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environmental Biology of Fishes, 58 (3): 237–275.
Benfey, T. J., Mccabe, L. E., and Pepin, P., 1997. Critical thermal maxima of diploid and triploid brook charr, Salvelinus fontinalis. Environmental Biology of Fishes, 49 (2): 259–264.
Brett, J. R., 1964. The respiratory metabolism and swimming performance of young sockeye salmon. Journal of the Fisheries Board of Canada, 21 (5): 1183–1226.
Brett, J. R., 1971. Energetic responses of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerkd). American Zoologist, 11 (1): 99–113.
Carline, R. F., and Machung, J. F., 2001. Critical thermal maxima of wild and domestic strains of trout. Transactions of the American Fisheries Society, 130 (6): 1211–1216.
Castro, V., Grisdale-Helland, B., Helland, S. J., Kristensen, T., Jørgensen, S. M., Helgerud, J., and Takle, H., 2011. Aerobic training stimulates growth and promotes disease resistance in Atlantic salmon (Salmo salar). Comparative Biochemistry and Physiology Part A Molecular and Integrative Physiology, 160 (2): 278–290.
Chapman, L. J., Chapman, C. A., Nordlie, F. G., and Rosenberger, A. E., 2002. Physiological refugia: Swamps, hypoxia tolerance and maintenance of fish diversity in the Lake Victoria region. Comparative Biochemistry and Physiology Part A Molecular and Integrative Physiology, 133 (3): 421–437.
Chown, S. L., Addo-Bediako, A., and Gaston, K. J., 2002. Physiological variation in insects: Large-scale patterns and their implications. Comparative Biochemistry and Physiology Part B Biochemistry and Molecular Biology, 131 (4): 587–602.
Cocking, A. W., 1959. The effects of high temperatures on roach (Rutilus Rutilus). Journal of Experimental Biology, 36 (1): 217–226.
Dhillon, R. S., Yao, L., Matey, V., Chen, B. J., Zhang, A. J., Cao, Z. D., and Fu, S. J., 2013. Interspecific differences in hypoxia-induced gill remodeling in carp. Physiological and Biochemical Zoology, 86 (6): 727–739.
Duarte, H., Tejedo, M., Katzenberger, M., Marangoni, F., and Baldo, D., 2012. Can amphibians take the heat? Vulnerability to climate warming in subtropical and temperate larval amphibian communities. Global Change Biology, 18: 412–421.
Elliott, J. M., and Elliott, J. A., 1995. The effect of the rate of temperature increase on the critical thermal maximum for parr of Atlantic salmon and brown trout. Journal of Fish Biology, 47 (5): 917–919.
Farrell, A. P., 2007. Cardiorespiratory performance during prolonged swimming tests with salmonids: A perspective on temperature effects and potential analytical pitfalls. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 362 (1487): 2017–2030.
Farrell, A. P., and Richards, J. G., 2009. Defining hypoxia: An integrative synthesis of the responses of fish to hypoxia. Fish Physiology, 27: 487–503.
Fields, R., Lowe, S. S., Kaminski, C., Whitt, G. S., and Philipp, D. P., 1987. Critical and chronic thermal maxima of northern and Florida largemouth bass and their reciprocal F1 and F2 hybrids. Transactions of the American Fisheries Society, 116 (6): 856–863.
Fu, S. J., Fu, C., Yan, G. J., Cao, Z. D., Zhang, A. J., and Pang, X., 2014. Interspecific variation in hypoxia tolerance, swimming performance and plasticity in cyprinids that prefer different habitats. Journal of Experimental Biology, 217 (4): 590–597.
Galbreath, P. F., Adams, N. D., and Martin, T. H., 2004. Influence of heating rate on measurement of time to thermal maximum in trout. Aquaculture, 241 (1-4): 587–599.
Gamperl, A. K., and Farrell, A. P., 2004. Cardiac plasticity in fishes: Environmental influences and intraspecific differences. Journal of Experimental Biology, 207 (15): 2539–2550.
Grande, M., and Andersen, S., 1991. Critical thermal maxima for young salmonids. Journal of Freshwater Ecology, 6 (3): 275–279.
Hopkin, R. S., Qari, S., Bowler, K., Hyde, D., and Cuculescu, M., 2006. Seasonal thermal tolerance in marine Crustacea. Journal of Experimental Marine Biology and Ecology, 331 (1): 74–81.
Hughes, G. M., 1973. Respiratory responses to hypoxia in fish. American Zoologist, 13 (2): 475–489.
Lee, R. M., and Rinne, J. N., 1980. Critical thermal maxima of five trout species in the southwestern United States. Transactions of the American Fisheries Society, 109 (6): 632–635.
Lutterschmidt, W. I., and Hutchison, V. H., 1997. The critical thermal maximum: History and critique. Canadian Journal of Zoology, 75 (10): 1561–1574.
Madeira, D., Narciso, L., Cabral, H. N., Diniz, M. S., and Vinagre, C., 2014a. Role of thermal niche in the cellular response to thermal stress: Lipid peroxidation and HSP70 expression in coastal crabs. Ecological Indicators, 36: 601–606.
Madeira, D., Vinagre, C., Costa, P. M., and Diniz, M. S., 2014b. Histopathological alterations, physiological limits, and molecular changes of juvenile Sparus aurata in response to thermal stress. Marine Ecology Progress, 505: 253–266.
Mette, R., Frode, O., Thomas, T., Albertk, I., and Rolferik, O., 2012. Effects of cyclic environmental hypoxia on physiology and feed intake of post-smolt Atlantic salmon: Initial responses and acclimation. Aquaculture, 326: 148–155.
Mora, C., and Maya, M. F., 2006. Effect of the rate of temperature increase of the dynamic method on the heat tolerance of fishes. Journal of Thermal Biology, 31 (4): 337–341.
Mora, C., and Ospína, A., 2001. Tolerance to high temperatures and potential impact of sea warming on reef fishes of Gorgona Island (tropical eastern Pacific). Marine Biology, 139 (4): 765–769.
Oppedal, F., Dempster, T., and Stien, L. H., 2011. Environmental drivers of Atlantic salmon behaviour in sea-cages: A review. Aquaculture, 311 (1): 1–18.
Pörtner, H., 2001. Climate change and temperature-dependent biogeography: Oxygen limitation of thermal tolerance in animals. The Science of Nature, 88 (4): 137–146.
Peck, L. S., Clark, M. S., Morley, S. A., Massey, A., and Rossetti, H., 2009. Animal temperature limits and ecological relevance: Effects of size, activity and rates of change. Functional Ecology, 23 (2): 248–256.
Powell, M. D., Fisk, D., and Nowak, B. F., 2000. Effects of graded hypoxia on Atlantic salmon infected with amoebic gill disease. Journal of Fish Biology, 57 (4): 1047–1057.
Remen, M., Aas, T. S., Vågseth, T., Torgersen, T., Olsen, R. E., Imsland, A., and Oppedal, F., 2014. Production performance of Atlantic salmon (Salmo salar L.) postsmolts in cyclic hypoxia, and following compensatory growth. Aquaculture Research, 45 (8): 1355–1366.
Remen, M., Oppedal, F., Imsland, A. K., Olsen, R. E., and Torgersen, T., 2013. Hypoxia tolerance thresholds for postsmolt Atlantic salmon: Dependency of temperature and hypoxia acclimation. Aquaculture, 416 (2): 41–47.
Rummer, J. L., Fangue, N. A., Jordan, H. L., Tiffany, B. N., Blansit, K. J., Galleher, S., Kirkpatrick, A., Kizlauskas, A. A., Pomory, C. M., and Bennett, W. A., 2009. Physiological tolerance to hyperthermia and hypoxia and effects on species richness and distribution of rockpool fishes of Loggerhead Key, Dry Tortugas National Park. Journal of Experimental Marine Biology and Ecology, 371 (2): 155–162.
Sadler, J., Wells, R. M., Pankhurst, P. M., and Pankhurst, N. W., 2000. Blood oxygen transport, rheology and haematological responses to confinement stress in diploid and triploid Atlantic salmon, Salmo salar. Aquaculture, 184 (3): 349–361.
Schurmann, H., and Steffensen, J. F., 1997. Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. Journal of Fish Biology, 50 (6): 1166–1180.
Seebacher, F., and Shine, R., 2004. Evaluating Thermoregulation in reptiles: The fallacy of the inappropriately applied method. Physiological and Biochemical Zoology, 77 (4): 688–695.
Steinhausen, M. F., Sandblom, E., Eliason, E. J., Verhille, C., and Farrell, A. P., 2008. The effect of acute temperature increases on the cardiorespiratory performance of resting and swimming sockeye salmon (Oncorhynchus nerka). Journal of Experimental Biology, 211 (24): 3915–3926.
Stevens, E. D., and Fry, F. E., 1974. Heat transfer and body temperatures in non-thermoregulatory teleosts. Canadian Journal of Zoology, 52 (9): 1137–1143.
Stevens, E. D., Sutterlin, A., and Cook, T., 1998. Respiratory metabolism and swimming performance in growth hormone transgenic Atlantic salmon. Canadian Journal of Fisheries and Aquatic Sciences, 55 (9): 2028–2035.
Terblanche, J. S., Sinclair, B. J., Jaco, K. C., Mcfarlane, M. L., and Chown, S. L., 2005. The effects of acclimation on thermal tolerance, desiccation resistance and metabolic rate in Chirodica chalcoptera (Coleoptera: Chrysomelidae). Journal of Insect Physiology, 51 (9): 1013–1023.
Thuy, N. H., Tien, L. A., Tuyet, P. N., Huong, D. T. T., Cong, N. V., Bayley, M., and Lefevre, S., 2010. Critical oxygen tension increases during digestion in the perch Perca fluviatilis. Journal of Fish Biology, 76 (4): 1025–1031.
van Raaij, M. T., Pit, D. S., Balm, P. H., Steffens, A. B., and Ge, V. D. T., 1996. Behavioral strategy and the physiological stress response in rainbow trout exposed to severe Hypoxia. Hormones and Behavior, 30 (1): 85–92.
Vinagre, C., Dias, M., Roma, J., Silva, A., Madeira, D., and Diniz, M. S., 2013. Critical thermal maxima of common rocky intertidal fish and shrimps–A preliminary assessment. Journal of Sea Research, 81: 10–12.
Vinagre, C., Leal, I., and Flores, A. A., 2015. Effect of warming rate on the critical thermal maxima of crabs, shrimp and fish. Journal of Thermal Biology, 47: 19–25.
Acknowledgements
We thank those who have critically review this manuscript and who helped us in this study at the Key Laboratory of Mariculture of Ministry of Education, Ocean University of China. This research was supported by the National Natural Science Foundation of China (Nos. 31572634 and 31702364), and Shandong Province Key Research and Development Plan (Nos. 2016CYJS04A01 and 2017CX GC0106).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, K., Dong, S., Zhou, Y. et al. Comparative Evaluation of Toleration to Heating and Hypoxia of Three Kinds of Salmonids. J. Ocean Univ. China 17, 1465–1472 (2018). https://doi.org/10.1007/s11802-018-3673-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-018-3673-9