Abstract
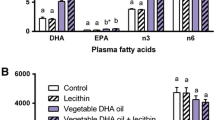
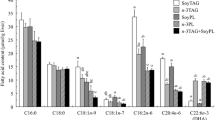
The bioavailability of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) depends on their chemical forms. This study investigated the long-term effects of DHA-bound triglyceride (TG-DHA), DHA-bound phospholipid (PL-DHA), and the combination of TG-DHA and egg yolk phospholipid (Egg-PL) on lipid metabolism in mice fed with a high-fat diet (fat levels of 22.5%). Male C57BL/6J mice were fed with different formulations containing 0.5% DHA, including TG-DHA, PL-DHA, and the combination of TG-DHA and Egg-PL, for 6 weeks. Serum, hepatic, and cerebral lipid concentrations and the fatty acid compositions of the liver and brain were determined. The concentrations of serum total triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), and hepatic TG in the PL-DHA group and the combination group were significantly lower than those in the high-fat (HF) group (P < 0.05). Atherogenic index (AI) of the PL-DHA group was significantly lower than that of the combination group (P < 0.05). Hepatic TC level in the combination group was significantly lower than that in the HF group (P < 0.05), but no significant difference was observed between the combination group and the PL-DHA group. Both the PL-DHA and the combination groups showed significantly increased DHA levels in the liver compared with the HF group (P < 0.05). However, there were no obvious increases in the cerebral DHA levels in all DHA diet groups. These results suggest that PL-DHA was superior to the combination of TG-DHA and Egg-PL in decreasing the AI. Long-term dietary supplementation with low amount of DHA (0.5%) may improve hepatic DHA levels, although cerebral DHA levels may not be enhanced.
Similar content being viewed by others
References
Awada, M., Meynier, A., Soulage, C. O., Hadji, L., Geloen, A., Viau, M., Ribourg, L., Benoit, B., Debard, C., Guichardant, M., Lagarde, M., Genot, C., and Michalski, M. C., 2013. n-3 PUFA added to high-fat diets affect differently adiposity and inflammation when carried by phospholipids or triacylglycerols in mice. Nutrition & Metabolism, 10: 23, DOI: 10.1186/1743-7075-10-23.
Bourre, J. M., Bonneil, M., Dumont, O., Piciotti, M., Calaf, R., Portugal, H., Nalbone, G., and Lafont, H., 1990. Effect of increasing amounts of dietary fish oil on brain and liver fatty composition. Biochimica et Biophysica Acta (BBA)–Lipids and Lipid Metabolism, 1043: 149–152, DOI: org/10.1016/0005-2760(90)90288-9.
Burri, L., Hoem, N., Banni, S., and Berge, K., 2012. Marine omega-3 phospholipids: metabolism and biological activities. International Journal of Molecular Sciences, 13: 15401–15419, DOI: 10.3390/ijms131115401.
Ding, N., Xue, Y., Tang, X., Sun, Z. M., Yanagita, T., Xue, C. H., and Wang, Y. M., 2013. Short-term effects of different fish oil formulations on tissue absorption of docosahexaenoic acid in mice fed high-and low-fat diets. Journal Oleo Science, 62: 883–891, DOI: org/10.5650/jos.62.883.
Folch, J., Lees, M., and Sloane Stanley, G. H., 1957. A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry, 226: 497–509.
Fukunaga, K., Hosomi, R., Fukao, M., Miyauchi, K., Kanda, S., Nishiyama, T., and Yoshida, M., 2016. Hypolipidemic effects of phospholipids (PL) containing n-3 polyunsaturated fatty acids (PUFA) are not dependent on esterification of n-3 PUFA to PL. Lipids, 51: 279–289, DOI: 10.1007/s11745-016-4118-0.
Harris, W. S., Miller, M., Tighe, A. P., Davidson, M. H., and Schaefer, E. J., 2008. Omega-3 fatty acids and coronary heart disease risk: Clinical and mechanistic perspectives. Atherosclerosis, 197: 12–24, DOI: org/10.1016/j.atherosclerosis.2007.11.008.
Hedengran, A., Szecsi, P. B., Dyerberg, J., Harris, W. S., and Stender, S., 2015. n-3 PUFA esterified to glycerol or as ethyl esters reduce non-fasting plasma triacylglycerol in subjects with hypertriglyceridemia: A randomized trial. Lipids, 50: 165–175, DOI: 10.1007/s11745-014-3968-6.
Higuchi, T., Shirai, N., and Suzuki, H., 2006. Effects of dietary herring roe lipids on plasma lipid, glucose, insulin, and adiponectin concentrations in mice. Journal of Agricultural and Food Chemistry, 54: 3750–3755, DOI: 10.1021/jf0531712.
Hiratsuka, S., Koizumi, K., Ooba, T., and Yokogoshi, H., 2009. Effects of dietary docosahexaenoic acid connecting phospholipids on the learning ability and fatty acid composition of the brain. Journal of Nutritional Science and Vitaminology, 55: 374–380, DOI: org/10.3177/jnsv.55.374.
Hung, P., Gu, J. Y., Kaku, S., Yunoki, S., Ohkura, K., Ikeda, I., Tachibana, H., Sugano, M., Yazawa, K., and Yamada, K., 2000. Dietary effects of eicosapentaenoic and docosahexaenoic acid esters on lipid metabolism and immune parameters in Sprague-Dawley rats. Bioscience, Biotechnology, and Biochemistry, 64: 2588–2593, DOI: org/10.1271/bbb.64.2588.
Kris-Etherton, P. M., Grieger, J. A., and Etherton, T. D., 2009. Dietary reference intakes for DHA and EPA. Prostaglandins, Leukotrienes and Essential Fatty Acids, 81: 99–104, DOI: org/10.1016/j.plefa.2009.05.011.
Lawson, L. D., and Hughes, B. G., 1988. Absorption of eicosapentaenoic acid and docosahexaenoic acid from fish oil triacylglycerols or fish oil ethyl esters co-ingested with a highfat meal. Biochemical and Biophysical Research Communications, 156: 960–963, DOI: org/10.1016/S0006-291X(88)80937-9.
Liu, X., Cui, J., Li, Z., Xu, J., Wang, J., Xue, C., and Wang, Y., 2014. Comparative study of DHA-enriched phospholipids and EPA-enriched phospholipids on metabolic disorders in dietinduced-obese C57BL/6J mice. European Journal of Lipid Science and Technology, 116: 255–265, DOI: 10.1002/ejlt.201300407.
Maki, K. C., Reeves, M. S., Farmer, M., Griinari, M., Berge, K., Vik, H., Hubacher, R., and Rains, T. M., 2009. Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutrition Research, 29: 609–615, DOI: org/10.1016/j.nutres.2009.09.004.
Masoodi, M., Kuda, O., Rossmeisl, M., Flachs, P., and Kopecky, J., 2015. Lipid signaling in adipose tissue: Connecting inflammation & metabolism. Biochimica et Biophysica Acta (BBA)–Molecular and Cell Biology of Lipids, 1851: 503–518, DOI: org/10.1016/j.bbalip.2014.09.023.
Nagao, K., Nakamitsu, K., Ishida, H., Yoshinaga, K., Nagai, T., Mizobe, H., Kojima, K., Yanagita, T., Beppu, F., and Gotoh, N., 2014. Comparison of the lipid-lowering effects of four different n-3 highly unsaturated fatty acids in HepG2 cells. Journal of Oleo Science, 63: 979–985, DOI: org/10.5650/jos.ess14118.
Riediger, N. D., Othman, R. A., Suh, M., and Moghadasian, M. H., 2009. A systemic review of the roles of n-3 fatty acids in health and disease. Journal of the American Dietetic Association, 109: 668–679, DOI: org/10.1016/j.jada.2008.12.022.
Robinson, J. G., and Stone, N. J., 2006. Antiatherosclerotic and antithrombotic effects of omega-3 fatty acids. The American Journal of Cardiology, 98: 39i–49i, DOI: org/10.1016/j.amjcard.2005.12.026.
Saravanan, P., Davidson, N. C., Schmidt, E. B., and Calder, P. C., 2010. Cardiovascular effects of marine omega-3 fatty acids. Lancet, 376: 540–550, DOI: org/10.1016/S0140-6736(10)60445-X.
Shirouchi, B., Nagao, K., Inoue, N., Ohkubo, T., Hibino, H., and Yanagita, T., 2007. Effect of dietary omega 3 phosphatidylcholine on obesity-related disorders in obese Otsuka Long-Evans Tokushima fatty rats. Journal of Agricultural and Food Chemistry, 55: 7170–7176, DOI: 10.1021/jf071225x.
Takahashi, K., and Inoue, Y., 2012. Marine by-product phospholipids as booster of medicinal compounds. Advances in Food and Nutrition Research, 65: 31–46.
Tanaka, Y., Ohkubo, T., Fukuda, N., and Hibino, H., 2003. Effect of molecular forms on distribution of docosahexaenoic acid into organs in mice. Journal Oleo Science, 52: 89–97, DOI: org/10.5650/jos.52.89.
Tandy, S., Chung, R. W., Wat, E., Kamili, A., Berge, K., Griinari, M., and Cohn, J. S., 2009. Dietary krill oil supplementation reduces hepatic steatosis, glycemia, and hypercholesterolemia in high-fat-fed mice. Journal of Agricultural and Food Chemistry, 57: 9339–9345, DOI: 10.1021/jf9016042.
Tang, X., Li, Z. J., Xu, J., Xue, Y., Li, J. Z., Wang, J. F., Yanagita, T., Xue, C. H., and Wang, Y. M., 2012. Short term effects of different omega-3 fatty acid formulation on lipid metabolism in mice fed high or low fat diet. Lipids in Health and Disease, 11: 70, DOI: 10.1186/1476-511X-11-70.
Yates, C. M., Calder, P. C., and Ed Rainger, G., 2014. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacology & Therapeutics, 141: 272–282, DOI: org/10.1016/j.pharmthera.2013.10.010.
Yavin, E., Brand, A., and Green, P., 2002. Docosahexaenoic acid abundance in the brain: A biodevice to combat oxidative stress. Nutritional Neuroscience, 5 (3): 149–157, DOI: 10.1080/10284150290003159.
Acknowledgements
This work was supported by the grants from the project supported by the State Key Program of National Natural Science of China (No. 31330060), the National Natural Science Foundation of China (Nos. 31301446, 31371757), and the Program for New Century Excellent Talents in University (No. NCET-13-0534).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Che, H., Cui, J., Wen, M. et al. Long-Term Effects of Docosahexaenoic Acid-Bound Phospholipids and the Combination of Docosahexaenoic Acid-Bound Triglyceride and Egg Yolk Phospholipid on Lipid Metabolism in Mice. J. Ocean Univ. China 17, 392–398 (2018). https://doi.org/10.1007/s11802-018-3444-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-018-3444-7