Abstract
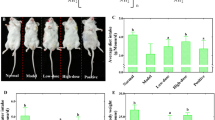
The effects of low-molecular-weight-chitosan (LMWC) on chronic renal failure (CRF) rats induced by adenine were investigated in vivo and in vitro. Chitosan were hydrolyzed using chitosanase at pH 6–7 and 37° for 24 h to obtain LMWC. In vitro, the effect of LMWC on the proliferation of renal tubular epithelial cells (RTEC) showed that it had no cytotoxic effect and could promote cell growth. For the in vivo experiment, chronic renal failure rats induced by adenine were randomly divided into control group, Niaoduqing group, and high-, medium- and low-dose LMWC groups. For each group, we detected serum creatinine (SCR), blood urea nitrogen (BUN), and total superoxide dismutase (T-SOD), glutathione oxidase (GSH-Px) activities of renal tissue, and obtained the ratio of kidney weight/body weight, pathological changes of kidney. The levels of serum SCR, BUN were higher in the adenine-induced rats than those in the control group, indicating that the rat chronic renal failure model worked successfully. The results after treatment showed that LMWC could reduce the SCR and BUN levels and enhance the activities/levels of T-SOD and GSH-PX in kidney compared to control group. Histopathological examination revealed that adenine-induced renal alterations were restored by LMWC at three tested dosages, especially at the low dosage of 100 mg kg−1 d−1.
Similar content being viewed by others
References
Amidi, M., Mastrobattista, E., Jiskoot, W., and Hennink, W. E., 2010. Chitosan-based delivery systems for protein therapeutic and antigens. Advanced Drug Delivery Reviews, 62(1): 59–82.
Arata, S., Ohmi, A., Mizukoshi, F., Baba, K., Ohno, K., Setoguchi, A., and Tsujimoto, H., 2005. Urinary transforming growth factor-beta 1 in feline chronic renal failture. Journal of Veterinary Medical Science, 67(12): 1253–1255.
Baek, K. S., Won, E. K., and Choung, S. Y., 2007. Effects of chitosan on serum cytokine levels in elderly subjects. Archives of Pharmacal Research, 30(12): 1550–1557.
Cartier, N., Lacave, R., Vallet, V., Hagege, J., Hellio, R., Robine, S., Pringault, E., Cluzeaud, F., Briand, P., and Kahn, A., 1993. Establishment of renal proximal tubule cell lines by targeted oncogenesis in transgenic mice using the Lpyruvate kinase-SV40(T) antigen hybrid gene. Journal of Cell Science, 104(Pt 3): 695–704.
Chang, Y.-M., Chang, C.-T., Huang, T.-C., Chen, S.-M., Lee, J.-A., and Chung, Y.-C., 2011. Effects of low molecular weight chitosans on aristolochic acid-induced renal lesions in mice. Food Chemistry, 129(4): 1751–1758.
Chung, M. J., Park, J. K., and Park, Y. I., 2012. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. International Immunopharmacology, 12(2): 453–459.
Guo, G., Morrissey, J., McCracken, R., Tolley, T, and Klahr, S., 1999. Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. American Journal of Physiology, 277(5 Pt 2): F766–772.
Howie, A. J., 2001. Handbook of Renal Biopsy Pathology. Springer, London, 1–244.
Jing, S. B., Li, L., Ji, D., Takiguchi, Y., and Yamaguchi, T., 1997. Effect of chitosan on renal function in patients with chronic renal failure. Journal of Pharmacy and Pharmacology, 49(7): 721–723.
Kumari, A., Yadav, S. K., and Yadav, S. C., 2010. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids and Surfaces B: Biointerfaces, 75(1): 1–18.
Maeda, Y., and Kimura, Y., 2004. Antitumor effects of various low-molecular-weight chitosans are due to increased natural killer activity of intestinal intraepithelial lymphocytes in sarcoma 180-bearing mice. The Journal of Nutrition, 134(4): 945–950.
Maezaki, Y., Tsuji, K., Nakagawai, Y., Kawai, Y., Akimoto, M., and Tsugita, T., 1993. Hypocholesterolemic effect of chitosan in adult males. Bioscience, Biotechnology and Biochemistry, 57: 1439–1444.
McGahren, W. J., Perkinson, G. A., Growich, J. A., Leese, R. A., and Ellestad, G. A., 1984. Chitosan by fermentation. Process Biochemistry, 19: 88–90.
Prashanth, K. V. H., and Tharanathan, R. N., 2007. Chitin/chitosan: Modifications and their unlimited application potential-An overview. Trends in Food Science & Technology, 18(3): 117–131.
Sinha, V. R., Singla, A. K., Wadhawan, S., Kaushik, R., Kumria, R., Bansal, K., and Dhawan, S., 2004. Chitosan icrospheres as a potential carrier for drugs. International Journal of Pharmaceutics, 274(1–2): 1–33.
Wang, D., Wu, X. F., and Jin, X. Y., 1999. Primary culture and passage of rat kidney tubular epithelial cells in rats. Chinese Journal of Experimental Surgery, 16(2): 179–180.
Wang, J., Zhang, Q. B., Jin, W. H., Niu, X. Z., and Zhang, H., 2011. Effects and mechanism of low molecular weight fucoidan in mitigating the peroxidative and renal damage induced by adenine. Carbohydrate Polymers, 84(1): 417–423.
Yin, H., Du, Y., and Zhang, J., 2009. Low molecular weight and oligomeric chitosans and their bioactivities. Current Topics in Medicinal Chemistry, 9(16): 1546–1559.
Yokozawa, T., Kanai, K., and Oura, H., 1977. Diurnal changes in uric-acid metabolism. Journal of the Agricultural Chemical Society of Japan, 51(9): 535–541.
Yokozawa, T., Zheng, P. D., Oura, H., and Koizumi, F., 1986. Animal-model of adenine-induced chronic-renal-failure in rats. Nephron, 44(3): 230–234.
Yuan, Z. X., Sun, X., Gong, T., Ding, H., Fu, Y., and Zhang, Z. R., 2007. Randomly 50% N-acetylated low molecular weight chitosan as a novel renal targeting carrier. Journal of Drug Target, 15(4): 269–278.
Yuan, Z. X., Zhang, Z. R., Zhu, D., Sun, X., Gong, T., Liu, J., and Luan, C. T., 2009. Specific renal uptake of randomly 50% N-acetylated low molecular weight chitosan. Molecular Pharmaceut, 6(1): 305–314.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhi, X., Han, B., Sui, X. et al. Effects of low-molecular-weight-chitosan on the adenine-induced chronic renal failure rats in vitro and in vivo . J. Ocean Univ. China 14, 97–104 (2015). https://doi.org/10.1007/s11802-015-2320-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-015-2320-y