Abstract
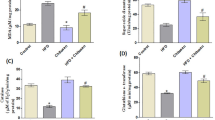
Sulfate chitosan derivatives have good solubility and therapeutic effect on the cell model of NAFLD. The aim of this study was to examine the therapeutic effect of sulfate chitosan derivatives on NAFLD. The male Wistar rats were orally fed high fat emulsion and received sulfate chitosan derivatives for 5 weeks to determine the pre-treatment effect of sulfate chitosan derivatives on NAFLD. To evaluate the therapeutic effect of sulfate chitosan derivatives on NAFLD, the rats were orally fed with high concentration emulsion for 5 weeks, followed by sulfate chitosan derivatives for 3 weeks. Histological analysis and biomedical assays showed that sulfate chitosan derivatives can dramatically prevent the development of hepatic steatosis in hepatocyte cells. In animal studies, pre-treatment and treatment with sulfate chitosan derivatives significantly protected against hepatic steatohepatitis induced by high fat diet according to histological analysis. Furthermore, increased TC, ALT, MDA, and LEP in NAFLD were significantly ameliorated by pre-treatment and treatment with sulfate chitosan derivatives. Furthermore, increased TG, AST, and TNF-α in NAFLD were significantly ameliorated by treatment with sulfate chitosan derivatives. Sulfate chitosan derivatives have good pre-treatment and therapeutic effect on NAFLD.
Similar content being viewed by others
References
Browning, J. D., and Horton, J. D., 2004. Molecular mediators of hepatic steatosis and liver injury. Journal of Clinical Investigation, 114(2): 147–152.
Clark, J. M., Brancati, F. L., and Diehl, A. M., 2003. The prevalence and etiology of elevated aminotransferase levels in the United States. The American Journal of Gastroenterology, 98(5): 960–967.
Deng, Z. B., Liu, Y., Liu, C., Xiang, X., Wang, J., Cheng, Z., Shah, S. V., Zhang, S., Zhang, L., Zhuang, X., Michalek, S., Grizzle, W. E., and Zhang, H. G., 2009. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology, 50(5): 1412–1420.
Fabbrini, E., Mohammed, B. S., Korenblat, K. M., Magkos, F., McCrea, J., Patterson, B. W., and Klein, S., 2010. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. The Journal of Clinical Endocrinology and Metabolism, 95(6): 2727–2735.
Gäbele, E., Dostert, K., Dorn, C., Patsenker, E., Stickel, F., and Hellerbrand, C., 2011. A new model of interactive effects of alcohol and high-fat diet on hepatic fibrosis. Alcoholism, Clinical and Experimental Research, 35(7): 1361–1367.
Jin, X., Yang, Y., Chen, K., Lv, Z., Zheng, L., Liu, Y., Chen, S., Yu, C., Jiang, X., Zhang, C., and Li, Y., 2009. HDMCP uncouples yeast mitochondrial respiration and alleviates steatosis in L02 and hepG2 cells by decreasing ATP and H2O2 levels: A novel mechanism for NAFLD. Journal of Hepatology, 50(5): 1019–1028.
Khor, E., and Lim, L. Y., 2003. Implantable applications of chitin and chitosan. Biomaterials, 24(13): 2339–2349.
Kim, D., Choi, S. Y., Park, E. H., Lee, W., Kang, J. H., Kim, W., Kim, Y. J., Yoon, J. H., Jeong, S. H., Lee, D. H., Lee, H., Larson, J., Therneau, T. M., and Kim, W. R., 2012. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology, 56(2): 605–613.
Kim, I., Seo, S. J., Moon, H. S., Yoo, M. K., Park, I. Y., Kim, B. C., and Cho, C. S., 2008. Chitosan and its derivatives for tissue engineering applications. Biotechnology Advances, 26(1): 1–21.
Kitade, M., Yoshiji, H., Kojima, H., Ikenaka, Y., Noguchi, R., Kaji, K., Yoshii, J., Yanase, K., Namisaki, T., Asada, K., Yamazaki, M., Tsujimoto, T., Akahane, T., Uemura, M., and Fukui, H., 2006. Leptin-mediated neovascularization is a prerequisite for progression of nonalcoholic steatohepatitis in rats. Hepatology, 44(4): 983–991.
Kumar, R., 2000. A review of chitin and chitosan applications. Reactive and Functional Polymers, 46(1): 1–27.
Ma, X., Hua, J., Mohamood, A. R., Hamad, A. R. A., Ravi, R., and Li, Z., 2007. A high-fat diet and regulatory t cells influence susceptibility to endotoxin-induced liver injury. Hepatology, 46(5): 1519–1529.
Manco, M., Marcellini, M., Giannone, G., and Nobili, V., 2007. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. American Journal of Clinical Pathology, 127(6): 954–960.
Masterton, G. S., Plevris, J. N., and Hayes, P. C., 2010. Review article: Omega-3 fatty acids — a promising novel therapy for non-alcoholic fatty liver disease. Alimentary Pharmacology and Therapeutics, 31(7): 679–692.
Mattar, S. G., Velcu, L. M., Rabinovitz, M., Demetris, A. J., Krasinskas, A. M., Barinas-Mitchell, E., Eid, G. M., Ramanathan, R., Taylor, D. S., and Schauer, P. R., 2005. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Transactions of the Meeting of the American Surgical Association, 123: 304–314.
Matteoni, C., Younossi, Z., Gramlich, T., Boparai, N., Liu, Y., and Mccullough, A., 1999. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology, 116(6): 1413–1419.
Nakao, Y., Yoshida, S., Matsunaga, S., Shindoh, N., Terada, Y., Nagai, K., Yamashita, J. K., Ganesan, A., van Soest, R. W. M., and Fusetani, N., 2006. Azumamides A-E: Histone deacetylase inhibitory cyclic tetrapeptides from the marine sponge mycale izuensis. Angewandte Chemie, 118(45): 7715–7719.
Oh, E., Kim, T. H., Sohn, Y. W., Kim, Y. S., Oh, Y. R., Cho, E. Y., Shim, S. Y., Shin, S. R., Han, A. L., Yoon, S. J., and Kim, H. C., 2011. Association of serum alanine aminotransferase and Γ-glutamyltransferase levels within the reference range with metabolic syndrome and nonalcoholic fatty liver disease. The Korean Journal of Hepatology, 17(1): 27–36.
Petta, S., Muratore, C., and Craxì, A., 2009. Non-alcoholic fatty liver disease pathogenesis: The present and the future. Digestive and Liver Disease, 41(9): 615–625.
Rafiq, N., and Younossi, Z. M., 2008. Effects of weight loss on nonalcoholic fatty liver disease. Seminars in Liver Disease, 28(4): 427–433.
Rosselli, M. S., Burgueño, A. L., Carabelli, J., Schuman, M., Pirola, C. J., and Sookoian, S., 2009. Losartan reduces liver expression of plasminogen activator inhibitor-1 (PAI-1) in a high fat-induced rat nonalcoholic fatty liver disease model. Atherosclerosis, 206(1): 119–126.
Taylor, S., and Harker, A., 2006. Modification of the ultrafiltration technique to overcome solubility and non-specific binding challenges associated with the measurement of plasma protein binding of corticosteroids. Journal of Pharmaceutical and Biomedical Analysis, 41(1): 299–303.
Tevar, A. D., Clarke, C. N., Schuster, R., Wang, J., Edwards, M., J., and Lentsch, A. B., 2011. The effect of hepatic ischemia reperfusion injury in a murine model of nonalcoholic steatohepatitis. The Journal of Surgical Research, 169(1): e7–14.
Uno, M., Kurita, S., Misu, H., Ando, H., Ota, T., Matsuzawa-Nagata, N., Kita, Y., Nabemoto, S., Akahori, H., Zen, Y., Nakanuma, Y., Kaneko, S., and Takamura, T., 2008. Tranilast, an antifibrogenic agent, ameliorates a dietary rat model of nonalcoholic steatohepatitis. Hepatology, 48(1): 109–118.
Wang, H., Chan, P. K., Pan, S. Y., Kwon, K. H., Ye, Y., Chu, J. H., Fong, W. F., Tsui, W. M. S., and Yu, Z. L., 2010. ERp57 is up-regulated in free fatty acids-induced steatotic L-02 cells and human nonalcoholic fatty livers. Journal of Cellular Biochemistry, 110(6): 1447–1456.
Yu, C., Lv, Z., Wang, Y., and Jiang, T., 2010. Study on the therapeutic effect of sulfated chitosan derivative on experimental fatty liver in rats. Periodical of Ocean University of China, 40(5): 27–32.
Zou, Y., Li, J., Lu, C., Wang, J., Ge, J., Huang, Y., Zhang, L., and Wang, Y., 2006. High-fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life Sciences, 79(11): 1100–1107.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, M., Wang, Y., Jiang, T. et al. Effects of sulfate chitosan derivatives on nonalcoholic fatty liver disease. J. Ocean Univ. China 13, 531–537 (2014). https://doi.org/10.1007/s11802-014-2511-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-014-2511-y