Abstract
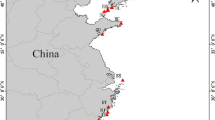
The genetic variation and differentiation of the razor clam Sinonovacula constricta distributed along the coast of China were studied through amplified fragment length polymorphism (AFLP) analysis. Six primer combinations generated 193 fragments. The H e values varied from 0.322 to 0.463 and the percentage of polymorphic loci ranged from 74.1% to 98.4%, which indicates a high level of genetic diversity. Cluster analysis by Nei’s pairwise distance grouped all specimens by geographical origins. AMOVA consistently showed that genetic variation among populations was 8.71%, and most of the variation came from the genetic variation within populations (91.29%). Genetic differentiation among the six populations was moderate; pairwise F ST ranged from 0.0282 to 0.1480, which indicated that S. constricta populations along the coast of China are genetically connected. Among all the six populations, the Beihai population is the mostly differentiated from the others, suggesting that Hainan Island and Leizhou Peninsula act as barriers to gene flow. All populations abide isolation by distance model as indicated by Mantel test, except for ZS (Zhoushan) and YQ (Yueqing) populations. Information obtained in this study will provide guidelines for conservation and fishery management of this species in the future.
Similar content being viewed by others
References
Ahren, D., Faedo, M., Rajashekar, B., and Tunlid, A., 2004. Low genetic diversity among isolates of the nematode-trapping fungus Duddingtonia flagrans: evidence for recent worldwide dispersion from a single common ancestor. Mycol. Res., 108: 1205–1214.
Baus, E., Darrock, D. J., and Bruford, M. W., 2005. Gene-flow patterns in Atlantic and Mediterranean populations of the Lusitanian sea star Asterina gibbosa. Mol. Ecol., 14: 3373–3382.
Bensch, S., and Åkesson, M., 2005. Ten years of AFLP in ecology and evolution: why so few animals? Mol. Ecol., 14: 2899–2914.
Benzie, J. A. H., Ballment, E., Forbes, A.T., Demetriades, N.T., Sugama, K., Haryanti, et al., 2002. Mitochondrial DNA variation in Indo-Pacific populations of the giant tiger prawn, Penaeus monodon. Mol. Ecol., 11: 2553–2569.
Cao, Q., 1998. Study on the population of Psendosciaena crocea on inshore of Zhanjiang. J. Zhangjiang Ocean Univ., 18 (2): 15–19 (in Chinese with English abstract).
Carlos, B., Ming, H. P., Carmen, R., Tom, H., Angela, K., and Ian, T., 2009. Genetic structure and population dynamics of a heteroecious plant pathogen Melampsora larici-epitea in short-rotation coppice willow plantations. Mol. Ecol., 18: 3006–3019.
Department of Fisheries (DOF), 2008. China Fisheries Statistic Yearbook 2007. China Agriculture Press, Beijing, 184–185.
Eline, D. L., Peter, A., and Hans, R. S., 2008. High variation and very low differentiation in wide ranging plains zebra (Equus quagga): insights from mtDNA and microsatellites. Mol. Ecol., 17: 2812–2824.
Excoffier, L., Laval, G., and Schneider, S., 2005. Arlequin version 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform., 1: 47–50.
Frankham, R., 1996. Relationship of genetic variation to population size in wildlife. Conserv. Biol., 10: 1500–1508.
Hartl, D. L., and Clark, A. G., 1997. Principles of Population Genetics. 3rd Edition. Sinauer Associates, Inc, Sunderland, MA, 121.
Hedgecock, D., 1986. Is gene flow from pelagic larval dispersal important in theadaptation and evolution of marine invertebrates? Bull. Mar. Sci., 39: 550–564.
Hedrick, P. W., 1985. Genetics of Populations. Jones and Bartlett, Boston, MA, 6–7.
Hindar, K. N., 1991. Genetic effects of cultured fish on natural fish population. Can. J. Fish. Aquat. Sci., 48: 945–957.
Juan, F. T., and Josefina, M., 2007. Identification of the razor clam species Ensis Arcuatus, E. siliqua, E. directus, E. macha, and Solen marginatus using PCR-RFLP analysis of the 5S rDNA region. J. Agric. Food Chem., 55: 7278–7282. doi:10.1021/jf0709855.
Kong, L. F., and Li, Q., 2009. Genetic evidence for the existence of cryptic species in an endangered clam Coelomactra antiquate. Mar. Biol., 156: 1507–1515.
Leander, D., Peter, C., Peter, B., Kristine, V. M., and Paul, G., 2009. A combined morphometric and AFLP based diversity study challenges the taxonomy of the European members of the complex Prunus L. section Prunus. Plant Syst. Evol., 279: 219–231.
Li, C. H., Li, T. W., Song, L. S., and Su, X. R., 2004. Genetic variations among four populations of Sinonovacula constricta by using random amplified polymorphic DNA. Fish. Sci., 23: 26–28 (in Chinese with English abstract).
Li, Q., Park, C., and Kijima, A., 2002. Isolation and characterization of microsatellite loci in the Pacific abalone, Haliotis discus hannai. J. Shellfish Res., 21: 811–815.
Liu, X. Q., Bao, Z. M., Hu, J. J., Wang, S., Zhan, A. B., Liu, H., et al., 2007, AFLP analysis revealed differences in genetic diversity of four natural populations of Manila clam (Ruditapes philippinarum) in China. Acta Oceanol. Sin., 26: 150–158.
Liu, Z. J., and Cordes, J. F., 2004. DNA marker technologies and their applications in aquaculture genetics. Aquaculture, 238: 1–37.
Miller, M. P., 1997. Tools for population genetic analysis (TFPGA) 1.3: A Windows program for the analysis of allozyme and molecular population genetic data. Available from http://bioweb.usu.edu/mpmbio/.
Mueller, U. G. and Wolfenbarger, L. L., 1999. AFLP genotyping and finger printing. Trends Ecol. Evol., 14: 389–394.
Nei, M., 1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics, 89: 583–590.
Niu, D. H., Li, J. L., and Liu, D. B., 2008a. Polymorphic microsatellite loci for population studies of the razor clam, Sinonovacula constricta. Conserv. Genet., 9: 1393–1394.
Niu, D. H., Li, J. L., Feng, B. B., and Liu, D. B., 2009. ISSR analysis on genetic structure of six Sinonovacula constricta populations. Chin. J. Appl. Environ. Biol., 15: 332–336 (in Chinese with English abstract).
Niu, D. H., Li, J. L., Shen, H. D., and Jiang, Z. Y., 2008b. Sequence variablitiy of mitochondrial DNA-COI gene fragment and population genetic structure of six Sinonovacula constricta populations. Acta Oceanol. Sin., 30: 109–116 (in Chinese with English abstract).
Parvin, S. S., Mohsen, M., Leila, P., Marianna, H., Damiano, A. S., Mostafa, P., et al., 2009. Analysis of the molecular variation between and within cultivated and wild Pistacia species using AFLPs. Tree Genet. Genomes, 5: 447–458.
Peakall, R., and Smouse, P. E., 2006. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes., 6: 288–295.
Slatkin, M., 1993. Isolation by distance in equilibrium and non-equilibrium populations. Evolution, 47: 264–279.
Thorp, J. P., 1982. The molecular dock hypothesis: Biochemical evolution, genetic differentiation and systmatics. Annu. Rev. Ecol. Syst., 13: 139–168.
Uthicke, S., and Benzie, J. A. H., 2003. Gene flow and population history in high dispersal marine invertebrates: mitochondrial DNA analysis of Holothuria nobilis (Echinodermata: Holothuroidea) populations from the Indo-Pacific. Mol. Ecol., 12: 2635–2648.
Vekemans, X., Beauwens, T., Lemaire, M., and Roldan-Ruiz, I., 2002. Data from amplified fragment length polymorphism (AFLP) markers show indication of size homoplasy and of a relationship between degree of homoplasy and fragment size. Mol. Ecol., 11: 139–151.
Vos, P., Hogers, R., Bleeker, M., Reijans, M., van de Lee, T., Hornes, M., et al., 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res., 23: 4407–4414.
Wang, W. X., and Xu, Z. Z., 1997. Larval swimming and post-larval drifting behavior in the infaunal bivalve Sinonovacula constricta. Mar. Ecol. Prog. Ser., 148: 71–81.
Wright, S., 1978. Evolution and the Genetics of Population, Variability Within and Among Natural Populations. The University of Chicago Press, Chicago, 580pp.
Yu, D. H., and Chu, K. H., 2006. Low genetic differentiation among widely separated populations of the pearl oyster Pinctada fucata as revealed by AFLP. J. Exp. Mar. Biol. Ecol., 333: 140–146.
Yu, H., Li, Q., and Yu, R. H., 2008. Microsatellite variation in the oyster Crassostrea plicatula along the coast of China. J. Ocean Univ. Chin., 7: 55–59. doi:10.1007/s11802-008-0055-8.
Zhang, Q., Allen, Jr., S. K., and Reece, K. S., 2005. Genetic variation in wild and hatchery stocks of Suminoe oyster (Crassostrea ariakensis) assessed by PCR-RFLP and microsatellite markers. Mar. Biotechnol., 7: 588–599.
Zhao, Y. M., Li, Q., Kong, L. F., Bao, Z. M., and Wang, R. C., 2007. Genetic diversity and divergence among clam Cyclina sinensis populations assessed using amplified fragment length polymorphism. Fish. Sci., 73: 1338–1343.
Zhivotovsky, L. A., 1999. Estimating population structure in diploids with multilocus dominant DNA markers. Mol. Ecol., 8: 907–913.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Li, Q. & Kong, L. Genetic variation and differentiation in wide ranging populations of razor clam (Sinonovacula constricta) inferred from AFLP markers. J. Ocean Univ. China 9, 297–302 (2010). https://doi.org/10.1007/s11802-010-1731-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-010-1731-z