Abstract
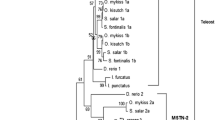
Myostatin or GDF-8, a member of the transforming growth factor-β (TGF-β) superfamily, has been demonstrated to be a negative regulator of skeletal muscle mass in mammals. In the present study, we obtained a 5.64 kb sequence of myostatin encoding gene and its promoter from largemouth bass (Micropterus salmoides). The myostatin encoding gene consisted of three exons (488 bp, 371 bp and 1779 bp, respectively) and two introns (390 bp and 855 bp, respectively). The intron-exon boundaries were conservative in comparison with those of mammalian myostatin encoding genes, whereas the size of introns was smaller than that of mammals. Sequence analysis of 1.569 kb of the largemouth bass myostatin gene promoter region revealed that it contained two TATA boxes, one CAAT box and nine putative E-boxes. Putative muscle growth response elements for myocyte enhancer factor 2 (MEF2), serum response factor (SRF), activator protein 1 (AP1), etc., and muscle-specific Mt binding site (MTBF) were also detected. Some of the transcription factor binding sites were conserved among five teleost species. This information will be useful for studying the transcriptional regulation of myostatin in fish.
Similar content being viewed by others
References
Brennan, T. J., T. Chakraborty, and E. N. Olson, 1991. Mutagenesis of the myogenin basic region identifies an ancient protein motif critical for activation of myogenesis. Proc. Natl. Acad. Sci. USA, 88: 5675–5679.
Chow, K. L., M. E. Hogan, and R. J. Schwartz, 1991. Phased cis-acting promoter elements interact at short distances to direct avian skeletal alpha-actin gene transcription. Proc. Natl. Acad. Sci. USA, 88(4): 1301–1305.
Crisà, A., C. Marchitelli, M. C. Savarese, and A. Valentini, 2003. Sequence analysis of myostatin promoter in cattle. Cytogenet. Genome Res., 102: 48–52.
Du, R., Y. Chen, X. An, X. Yang, and Y. Ma, L. Zhang, et al., 2005. Cloning and sequence analysis of myostatin promoter in sheep. DNA Seq., 16(6): 412–417.
Grobet, L., L. J. Martin, D. Poncelet, and D. Pirottin, 1997. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet., 17: 71–74.
Grobet, L., D. Poncelet, L. J. Royo, B. Brouwers, and D. Pirottin, C. Michaux, et al., 1998. Molecular definition of an allelic series of mutations disrupting the myostatin function and causing double-muscling in cattle. Mamm. Genome, 9:210–213.
Hochschild, A., and M. Ptashne, 1986. Cooperative binding of lambda repressors to sites separated by integral turns of the DNA helix. Cell, 44(5):681–687.
Kambadur, R., M. Sharma, T. P. L. Smith, and J. J. Bass, 1997. Mutations in myostatin (GDF8) in double-muscled Belgian blue and Piedmontese cattle. Genome Res., 7: 910–915.
Lassar, A. B., J. N. Buskin, D. Lockshon, R. L. Davis, and S. Apone, S. D. Hauschka, et al., 1989. MyoD is a sequence specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell, 58: 823–831.
Lee, S. J., and A. C. McPherron, 2001. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA, 98(16): 9306–9311.
Lin, H., K. Yutzey, and S. F. Konieczny, 1991. Muscle-specific expression of the troponin I gene requires interactions between helixloop-helixloop-helix muscle regulatory factors and ubiquitous transcription factors. Mol. Cell Biol., 11:267–280.
Ma, K., C. Mallidis, J. Artaza, W. E. Taylor, and N. Gonzalez-Cadavid, S. Bhasin, et al., 2001. Characterization of 5′-regulatory region of human myostatin gene: regulation by dexamethasone in vitro. Am. J. Physiol. Endocrinol. Metab., 281: 1128–1136.
Maccatrozzo, L., L. Bargelloni, G. Radaelli, F. Mascarello, and T. Patarnello, 2001. Characterization of the myostatin gene in the gilthead seabream (Sparus aurata): Sequence, genomic structure, and expression pattern. Mar. Biotechnol., 3:224–230.
Maccatrozzo, L., L. Bargelloni, P. Patarnello, G. Radaelli, and F. Mascarello, and T. Patarnello, 2002. Characterization of the myostatin gene and a linked microsatellite marker in shi drum (Umbrina cirrosa, Sciaenidae). Aquaculture, 205(1): 49–60.
Malik, S., C. F. Huang, and J. Schmidt, 1995. The role of the CANNTG promoter element (E box) and the myocyte-enhancer-binding-factor-2 (MEF-2) site in the transcriptional regulation of the chick myogenin gene. Eur. J. Biochem., 230: 88–96.
McPherron, A. C., A. M. Lawler, and S. J. Lee, 1997a. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature, 387: 83–90.
McPherron, A. C., and S. J. Lee, 1997b. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA, 94(23): 12457–12461.
Rios, R., I. Carneiro, V. M. Arce, and J. Devesa, 2002. Myostatin is an inhibitor of myogenic differentiation. Am. J. Physiol. Cell Physiol., 282: 993–999.
Roberts, S. B., and F. W. Goetz, 2001. Differential skeletal muscle expressionof myostatin across teleost species and the isolation of multiple myostatin isoform. FEBS Lett., 491:212–216.
Rogers, B. D., and G. W. Weber, 2001. Sequence conservation among fish myostatin orthologues and the characterization of two additional cDNA clones from Marone soxitilis and Marone americana. Comp. Biochem. Physiol., 129: 597–603.
Salerno, M. S., M. Thomas, D. Forbes, T. Watson, R. Kambadur, M. Sharma, et al., 2004. Molecular analysis of fiber type-specific expression of murine myostatin promoter. Am. J. Physiol. Cell Physiol., 287: C1031–C1040.
Spiller, M. P., R. Kambadur, F. Jeanplong, M. Thomas, J. K. Martyn, J. J. Bass, et al., 2002. The Myostatin gene is a downstream target gene of basic helix-loop-helix transcription factor MyoD. Mol. Cell Biol., 22: 7066–7082.
Takahashi, K., M. Vigneron, H. Matthes, A. Wildeman, and M. Zenke, and P. Chambon, 1986. Requirement of stereospecific alignments for initiation from the simian virus 40 early promoter. Nature, 319:121–126.
Taylor, W. E., S. Bhasin, J. Artaza, F. Byhower, M. Azam, Jr. Willard, et al., 2001. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am. J. Physiol. Endocrinol. Metab., 280: 221–228.
Thomas, M., B. Langley, C. Berry, M. Sharma, S. Kirk, J. Bass, et al., 2000. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J. Biol. Chem., 275(51): 40 235–40 243.
Xu, C., G. Wu, Y. Zohar, and S. Du, 2003. Analysis of myostatin gene structure, expression and function in zebrafish. J. Exp. Biol., 206: 4067–4079.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S., Bai, J. & Wang, L. Cloning and characterization of largemouth bass (Micropterus salmoides) myostatin encoding gene and its promoter. J. Ocean Univ. China 7, 304–310 (2008). https://doi.org/10.1007/s11802-008-0304-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-008-0304-x