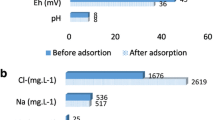
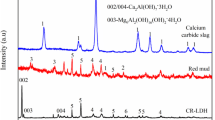
Abstract
A spent fluid catalytic cracking (FCC) catalyst containing lanthanum (La) was used as a novel adsorbent for phosphorus (P) in simulated wastewater. The experiments were conducted in a batch system to optimize the operation variables, including pH, calcination temperature, shaking time, solid-liquid ratio, and reaction temperature under three initial P-concentrations (C0 = 0.5, 1.0, and 5.0 mg/L). Orthogonal analysis was used to determine that the initial P-concentration was the most important parameter for P removal. The P-removal rate exceeded 99% and the spent FCC catalyst was more suitable for use in low P-concentration wastewater (C0 <5.0 mg/L). Isotherms, thermodynamics and dynamics of adsorption are used to analyze the mechanism of phosphorus removal. The results show that the adsorption is an endothermic reaction with high affinity and poor reversibility, which indicates a low risk of second releasing of phosphate. Moreover, chemical and physical adsorption coexist in this adsorption process with LaPO4 and KH2PO4 formed on the spent FCC catalyst as the adsorption product. These results demonstrate that the spent FCC catalyst containing La is a potential adsorbent for P-removal from wastewater, which allows recycling of the spent FCC catalyst to improve the quality of water body.

Similar content being viewed by others
References
Al–Khattaf S (2002). The influence of Y–zeolite unit cell size on the performance of FCC catalysts during gas oil catalytic cracking. Applied Catalysis A, General, 231(1): 293–306
Babatunde A O, Zhao Y Q (2010). Equilibrium and kinetic analysis of phosphorus adsorption from aqueous solution using waste alum sludge. Journal of Hazardous Materials, 184(1–3): 746–752
Bauer D, Diamond D, Li J, Sandalow D, Telleen P,Wanner B (2010). U. S. Department of Energy Critical Materials Strategy, U. S.
Bekçi Z, Seki Y, Kadir Yurdakoç M (2007). A study of equilibrium and FTIR, SEM/EDS analysis of trimethoprim adsorption onto K10. Journal of Molecular Structure, 827(1): 67–74
Binnemans K, Jones P T, Blanpain B, Van Gerven T, Yang Y, Walton A, Buchert M (2013). Recycling of rare earths: A critical review. Journal of Cleaner Production, 51: 1–22
Brigante M, Schulz P C (2012). Adsorption of the antibiotic minocycline on cerium(IV) oxide: Effect of pH, ionic strength and temperature. Microporous and Mesoporous Materials, 156: 138–144
Cho S, Luong T T, Lee D, Oh Y K, Lee T (2011). Reuse of effluent water from a municipal wastewater treatment plant in microalgae cultivation for biofuel production. Bioresource Technology, 102(18): 8639–8645
Ding W, Huang X (2002). Progress of studies on phosphorus removal from wastewater by adsorbents. Technigues and Equipment for Enviro. poll. 3(10): 23–27
Guaya D, Valderrama C, Farran A, Armijos C, Cortina J L (2015). Simultaneous phosphate and ammonium removal from aqueous solution by a hydrated aluminum oxide modified natural zeolite. Chemical Engineering Journal, 271: 204–213
Horsfall M Jr, Abia A A, Spiff A I (2006). Kinetic studies on the adsorption of Cd2+, Cu2+ and Zn2+ ions from aqueous solutions by cassava (Manihot sculenta Cranz) tuber bark waste. Bioresource Technology, 97(2): 283–291
Hrenovic J, Rozic M, Sekovanic L, Anic–Vucinic A (2008). Interaction of surfactant–modified zeolites and phosphate accumulating bacteria. Journal of Hazardous Materials, 156(1–3): 576–582
Huang H, Xiao D, Pang R, Han C, Ding L (2014). Simultaneous removal of nutrients from simulated swine wastewater by adsorption of modified zeolite combined with struvite crystallization. Chemical Engineering Journal, 256: 431–438
Huo H, Lin H, Dong Y, Cheng H, Wang H, Cao L (2012). Ammonianitrogen and phosphates sorption from simulated reclaimed waters by modified clinoptilolite. Journal of Hazardous Materials, 229–230: 292–297
Ihm S K, Chung M J, Park K Y (1988). Activity difference between the internal and external sulfonic groups of macroreticular ion–exchange resin catalysts in isobutylene hydration. Industrial & Engineering Chemistry Research, 27(1): 41–45
JaycockMJ, Parfitt G D (1981). Chemistry of Interfaces. Halstead Press, John Wiley and Sons, 718
Jiang L, Lin H, Jia X, Guo L (2010). Preparation of compounded adsorption of phosphorus removal with zeolite and its performance. Metal Mine, 10: 154–158
Johan E, Shukla E A, Matsue N, Henmi T (2013). Fe–treated artificial zeolite as an adsorbent for anionic and cationic pollutants. Procedia Environmental Sciences, 17: 285–290
Karczmarczyk A, Bus A (2014). Testing of reactive materials for phosphorus removal from water and waste water–comparative study. Annals of Warsaw University of Life Sciences–SGGW Land Reclamation, 46(1): 57–67
Li X, Hu H Y, Yang J (2010). Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga, Scenedesmus sp. LX1, growing in secondary effluent. New Biotechnology, 27(1): 59–63
Lorenc–Grabowska E, Gryglewicz G (2007). Adsorption characteristics of Congo Red on coal–based mesoporous activated carbon. Dyes and Pigments, 74(1): 34–40
McKay G (1983). The adsorption of dyestuffs from aqueous solution using activated carbon: Analytical solution for batch adsorption based on external mass transfer and pore diffusion. Chemical Engineering Journal, 27(3): 187–196
McKay G, Blair H S, Gardner J R (1982). Adsorption of dyes on chitin. I. Equilibrium studies. Journal of Applied Polymer Science, 27(8): 3043–3057
Ministry of Environmental Protection of the People's Republic of China (2007a). Identification standards for hazardous waste–identification for extraction toxicity. GB5085.3–2007
Ministry of Environmental Protection of the People's Republic of China (2007b). Solid waste–extraction procedure for leaching toxicitysulphuric acid & nitric acid method. HJ/T299–2007
Ning P, Bart H J, Li B, Lu X, Zhang Y (2008). Phosphate removal from wastewater by model–La(III) zeolite adsorbents. Journal of Environmental Sciences (China), 20(6): 670–674
Pavan F A, Dias S L P, Lima E C, Benvenutti E V (2008). Removal of Congo Red from aqueous solution by anilinepropylsilica xerogel. Dyes and Pigments, 76(1): 64–69
Ramli N A S, Amin N A S (2016). Kinetic study of glucose conversion to levulinic acid over Fe/HY zeolite catalyst. Chemical Engineering Journal, 283: 150–159
Rashidi Nodeh H, Sereshti H, Zamiri Afsharian E, Nouri N (2017). Enhanced removal of phosphate and nitrate ions from aqueous media using nanosized lanthanum hydrous doped on magnetic graphene nanocomposite. Journal of Environmental Management, 197: 265–274
Sanga S, Nishimura Y (1976). Sewer waste water treating agent produced from waste cracking catalyst. U. S. Patent No. 3, 960, 760
Shi W, He B, Cao Y, Li J, Yan F, Cui Z, Zou Z, Guo S, Qian X (2013). Continuous esterification to produce biodiesel by SPES/PES/NWF composite catalytic membrane in flow–through membrane reactor: Experimental and kinetic studies. Bioresource Technology, 129: 100–107
Singh D (2000). Studies of the adsorption thermodynamics of oxamyl on fly ash. Adsorption Science and Technology, 18(8): 741–748
Song Q, Fang Y, Liu Z, Li L, Wang Y, Liang J, Huang Y, Lin J, Hu L, Zhang J, Tang C (2017). The performance of porous hexagonal BN in high adsorption capacity towards antibiotics pollutants from aqueous solution. Chemical Engineering Journal, 325: 71–79
Streat M, Hellgardt K, Newton N L R (2008). Hydrous ferric oxide as an adsorbent in water treatment: Part 3: Batch and mini–column adsorption of arsenic, phosphorus, fluorine and cadmium ions. Process Safety and Environmental Protection, 86(1): 21–30
Tang Y, Chen F, Zhang H (1998). Adsorption of Pb2+, Cu2+ and Zn2+ ions on to waste fluidized catalytic cracking (FCC) catalyst. Adsorption Science and Technology, 16(8): 595–606
Vimonses V, Lei S, Jin B, Chow C W K, Saint C (2009). Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chemical Engineering Journal, 148(2): 354–364
Wallenstein D, Kanz B, Haas A (2000). Influence of coke deactivation and vanadium and nickel contamination on the performance of low ZSM–5 levels in FCC catalysts. Applied Catalysis A, General, 192 (1): 105–123
Weber W J, Morris J C (1963). Kinetics of adsorption on carbon from solution. Journal of the Sanitary Engineering Division, 89(2): 31–60
Weisz P B, Hicks J S (1962). The behaviour of porous catalyst particles in view of internal mass and heat diffusion effects. Chemical Engineering Science, 17(4): 265–275
Weng C H, Lin Y T, Tzeng T W (2009). Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. Journal of Hazardous Materials, 170(1): 417–424
Wu B, Fang L, Fortner J D, Guan X, Lo I M C (2017). Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites. Water Research, 126: 179–188
Xie J, Wang Z, Fang D, Li C, Wu D (2014). Green synthesis of a novel hybrid sorbent of zeolite/lanthanum hydroxide and its application in the removal and recovery of phosphate from water. Journal of Colloid and Interface Science, 423: 13–19
Yoo J S (1998). Metal recovery and rejuvenation of metal–loaded spent catalysts. Catalysis Today, 44(1): 27–46
Zhang D, Niu H, Zhang X, Meng Z, Cai Y (2011). Strong adsorption of chlorotetracycline on magnetite nanoparticles. Journal of Hazardous Materials, 192(3): 1088–1093
Zhang H, Xiao R, Wang D, Zhong Z, Song M, Pan Q, He G (2009). Catalytic fast pyrolysis of biomass in a fluidized bed with fresh and spent fluidized catalytic cracking (FCC). Catalysts. Energy & Fuels, 23(12): 6199–6206
Zhao Z, Qiu Z, Yang J, Lu S, Cao L, Zhang W, Xu Y (2017). Recovery of rare earth elements from spent fluid catalytic cracking catalysts using leaching and solvent extraction techniques. Hydrometallurgy, 167: 183–188
Zheng S, Huang S, Qian D, Tang K, Yi J (2010). Modification of FCC waste catalyst and its adsorption performance for heavy metal ions. Acta Petrolei Sinica, 26(4): 612–616
Acknowledgement
Supplementary material is available in the online version of this article at https://doi.org/10.1007/s11783-018-1082-3 and is accessible for authorized users.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, Z., Qiu, Z., Yang, J. et al. Investigation of phosphate adsorption from an aqueous solution using spent fluid catalytic cracking catalyst containing lanthanum. Front. Environ. Sci. Eng. 12, 15 (2018). https://doi.org/10.1007/s11783-018-1082-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-018-1082-3