Abstract
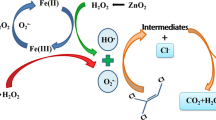
The degradation of selected chlorinated aliphatic hydrocarbons (CAHs) exemplified by trichloroethylene (TCE), 1,1-dichloroethylene (DCE), and chloroform (CF) was investigated with Fenton oxidation process. The results indicate that the degradation rate was primarily affected by the chemical structures of organic contaminants. Hydroxyl radicals (·OH) preferred to attack the organic contaminants with an electron-rich structure such as chlorinated alkenes (i.e., TCE and DCE). The dosing mode of Fenton’s reagent, particularly of Fe2+, significantly affected the degradation efficiency of studied organic compound. A new “time-squared” kinetic model, C = C o exp(−k obs t 2), was developed to express the degradation kinetics of selected CAHs. This model was applicable to TCE and DCE, but inapplicable to CF due to their varied reaction rate constants towards ?OH. Chloride release was monitored to examine the degree of dechlorination during the oxidation of selected CAHs. TCE was more easily dechlorinated thanDCE and CF.Dichloroacetic acid (DCAA) was identified as the major reaction intermediate in the oxidation of TCE, which could be completely removed as the reaction proceeded. No reaction intermediates or byproducts were identified in the oxidation of DCE and CF. Based on the identified intermediate, the reaction mechanism of TCE with Fenton’s reagent was proposed.
Similar content being viewed by others
References
Vogel T M, Criddle C S, McCarty P L. Transformations of halogenated aliphatic compounds: oxidation, reduction, substitution, and dehydrohalogenation reactions occur abiotically or in microbial and mammalian systems. Environmental Science & Technology, 1987, 21(8): 722–736
Watts R J. Hazardous Wastes: Sources, Pathways, Receptors. New York: John Wiley & Sons, 1998
Higgins T E. Hazardous Waste Minimization Handbook. Chelsea: Lewis Publishers, 1989
Mackay D M, Smith L A. Agricultural chemicals in groundwater: Monitoring and management in California. Journal of Soil and Water Conservation, 1990, 45(2): 253–255
ATSDR Biannual Report to Congress (10/17/1986-9/30/1988). Agency for Toxic Substances and Disease Registry, U.S. Public Health Service, Atlanta, GA, 1989
Keith L H, Telliard W A. Priority pollutants I-a perspective view. Environmental Science & Technology, 1979, 13(4): 416–423
El-Farhan Y H, Scow K M, de Jonge L W, Rolston D E, Moldrup P. Coupling transport and biodegradation of toluene and trichloroethylene in unsaturated soils. Water Resources Research, 1998, 34(3): 437–445
Heron G, Van Zutphen M, Christensen T H, Enfield C G. Soil heating for enhanced remediation of chlorinated solvents: a laboratory study on resistive heating and vapor extraction in a silty, low-permeable soil contaminated with trichloroethylene. Environmental Science & Technology, 1998, 32(10): 1474–1481
Kawala Z, Atamanczuk T. Microwave-enhanced thermal decontamination of soil. Environmental Science & Technology, 1998, 32(17): 2602–2607
Huang C P, Dong C D, Tang Z H. Advanced chemical oxidation: Its present role and future potential in hazardous waste treatment. Waste Management, 1993, 13(5/7): 361–377
Burris D R, Delcomyn C A, Deng B L, Buck L E, Hatfield K. Kinetics of tetrachloroethylene-reductive dechlorination catalyzed by vitamin B12. Environmental Toxicology and Chemistry, 1998, 17(9): 1681–1688
Ho S V, Athmer C, Sheridan P W, Hughes B M, Orth R, McKenzie D, Brodsky P H, Shapiro A, Thornton R, Salvo J, Schultz D, Landis R, Griffith R, Shoemaker S. The lasagna technology for in situ soil remediation. 1. Small field test. Environmental Science & Technology, 1999, 33(7): 1086–1091
Ho S V, Athmer C, Sheridan P W, Hughes B M, Orth R, McKenzie D, Brodsky P H, Shapiro A, Sivaves T M, Salvo J, Schultz D, Landis R, Griffith R, Shoemaker S. The lasagna technology for in situ soil remediation. 2. Large field test. Environmental Science & Technology, 1999, 33(7): 1092–1099
Gotpagar J, Grulke E, Tsang T, Bhattacharyya D. Reductive dehalogenation of trichloroethylene using zero-valent iron. Environmental Progress, 1997, 16(2): 137–143
Arnold W A, Roberts A L. Pathways of chlorinated ethylene and chlorinated acetylene reaction with Zn (0). Environmental Science & Technology, 1998, 32(19): 3017–3025
Cooper W J, Meacham D E, Nickelsen M G, Lin K, Ford D B, Kurucz C N, Waite T D. The removal of trichloroethylene (TCE) and tetrachloroethylene (PCE) from aqueous-solution using high-energy electrons. Journal of the Air & Waste Management Association, 1993, 43(10): 1358–1366
Bhatnagar A, Cheung H M. Sonochemical destruction of chlorinated C1 and C2 volatile organic compounds in dilute aqueous solution. Environmental Science & Technology, 1994, 28(8): 1481–1486
Crittenden J C, Liu J, Hand D W, Perram D L. Photocatalytic oxidation of chlorinated hydrocarbons in water. Water Research, 1997, 31(3): 429–438
Ollis D F, Hsiao C Y, Budiman L, Lee C L. Heterogeneous photoassisted catalysis: conversions of perchloroethylene, dichloroethane, chloroacetic acids, and chlorobenzenes. Journal of Catalysis, 1984, 88(1): 89–96
Amama P B, Itoh K, Murabayashi M. Effect of RuO2 deposition on the activity of TiO2: Photocatalytic oxidation of trichloroethylene in aqueous phase. Journal of Materials Science, 2004, 39: 4349–4351
Glaze W H, Kenneke J F, Ferry J L. Chlorinated byproducts from the TiO2-mediated photodegradation of trichloroethylene and tetrachloroethylene in water. Environmental Science & Technology, 1993, 27(1): 177–184
Bull R J, Sanchez I M, Nelson M A, Larson J L, Lansing A J. Liver tumor induction in B6C3F1 mice by dichloroacetate and trichloroacetate. Toxicology, 1990, 63(3): 341–359
Ravikumar J X, Gurol M D. Chemical oxidation of chlorinated organics by hydrogen peroxide in the presence of sand. Environmental Science & Technology, 1994, 28(3): 394–400
Chen G, Hoag G E, Chedda P, Nadim F, Woody B A, Dobbs G M. The mechanism and applicability of in situ oxidation of trichloroethylene with Fenton’s reagent. Journal of Hazardous Materials, 2001, B87: 171–186
Tang W Z, Huang C P. Stoichiometry of Fenton’s reagent in the oxidation of chlorinated aliphatic organic pollutants. Environmental Technology, 1997, 18(1): 13–23
Tang W Z, Tassos S. Oxidation kinetics and mechanisms of trihalomethanes by Fenton’s reagent. Water Research, 1997, 31(5): 1117–1125
Liang C, Bruell C J. Thermally activated persulfate oxidation of trichloroethylene: Experimental investigation of reaction orders. Industrial Engineering Chemical Research, 2008, 47: 2912–2918
Schwarzenbach R P, Gschwend P M, Imboden D M. Environmental Organic Chemistry. New York: John Wiley & Sons, 1993
Fales H M, Jaouni T M, Babashak J F. Simple device for preparing ethereal diazomethane without resorting to codistillation. Analytical Chemistry, 1973, 45(13): 2302–2303
Knapp D R. Handbook of Analytical Derivatization Reactions. New York: John Wiley & Sons, 1979
Qiang Z M, Chang J H, Huang C P, Cha D. Oxidation of selected polycyclic aromatic hydrocarbons by the Fenton’s reagent: effect of major factors including organic solvent. In: Heineman W R, Eller P G, eds. Nuclear Site Remediation: First Accomplishments of the Environmental Management Science Program. Washington, DC: American Chemical Society, 2000, 187–209
Walling C. Fenton’s reagent revisited. Accounts of Chemical Research, 1975, 8: 125–131
Sedlak D L, Andren A W. Oxidation of chlorobenzene with Fenton’s reagent. Environmental Science & Technology, 1991, 25(4): 777–782
Beltran F J, Gonzalez M, Rivas F J, Alvarez P. Fenton reagent advanced oxidation of polynuclear aromatic hydrocarbons in water. Water Air & Soil Pollution, 1998, 105(3–4): 685–700
Getoff N. Radiation-degradation and photoinduced-degradation of pollutants in water—A comparative-study. Radiation Physics and Chemistry, 1991, 37(5–6): 673–680
Köster R, Asmus K D. Die Reaktionen chlorierter Äthylene mit hydrotisierten Elektronen und OH-Radikalen in wässriger Lösung. Z. Naturforsch, 1971, 26b: 1108–1116
Haag W R, Yao C C D. Rate constants for reaction of hydroxyl radicals with several drinking-water contaminants. Environmental Science & Technology, 1992, 26(5): 1005–1013
Chen J R, Xu X W, Lee A S, Yen T F. A feasibility study of dechlorination of chloroform in water by ultrasound in the presence of hydrogen peroxide. Environmental Technology, 1990, 11(9): 829–836
Pignatello J J, Liu D, Huston P. Evidence for an additional oxidant in the photoassisted Fenton reaction. Environmental Science & Technology, 1999, 33(11): 1832–1839
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiang, Z., Ben, W. & Huang, CP. Fenton process for degradation of selected chlorinated aliphatic hydrocarbons exemplified by trichloroethylene, 1,1-dichloroethylene and chloroform. Front. Environ. Sci. Eng. China 2, 397–409 (2008). https://doi.org/10.1007/s11783-008-0074-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-008-0074-0