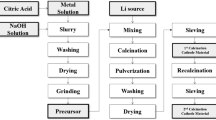
Abstract
The constructed potential-pH diagrams of Li-Ni (Co, Mn)-H2O system indicate that the LiNiO2, LiCoO2 and LiMnO2 are thermodynamically stable in aqueous solution within the temperature range of 25-200 °C and the activity range of 0.01-1.00. A predominant co-region of LiNiO2, LiCoO2 and LiMnO2 oxides (Li-Ni-Co-Mncomposite oxide) is found in the Li-Ni-Co-Mn-H2O potential-pH diagrams, in which the co-precipitation region expands towards lower pH with rising temperature, indicating the enhanced possibility of synthesizing Li-Ni-Co-Mn composite oxide in aqueous solution. The experimental results prove that it is feasible to prepare the LiNi0.5Co0.2Mn0.3O2 cathode materials (NCM523) by an aqueous routine. The as-prepared lithiated precursor and NCM523 both inherit the spherical morphology of the hydroxide precursor and the obtained NCM523 has a hexagonal α-NaFeO2 structure with good crystallinity. It is reasonable to conclude that the aqueous routine for preparing Ncm cathode materials is a promising method with the guidance of the reliable potential-pH diagrams to some extent.
摘要
本文构建的Li-Ni(Co, Mn)-H2O 系电位−pH 图从热力学角度表明,在25≈250 °C 温度范围和 0.01~1.00 活度范围内,LiNiO2, LiCoO2 和LiMnO2 可以稳定存在于水溶液中。在Li-Ni-Co-Mn-H2O 系 电位−pH 图中,同时出现了LiNiO2、LiCoO2 和LiMnO2 氧化物(即Li-Ni-Co-Mn 复合氧化物)的优势 区,并且该共沉淀优势区随温度的升高向低pH 方向扩大,表明在水溶液中合成Li-Ni-Co-Mn 复合氧 化物是可能的。在此基础上,本文通过实验采用湿法过程合成了LiNi0.5Co0.2Mn0.3O2 (NCM523)正极材 料,证明了上述理论的可行性。合成的锂化前驱体和NCM523 材料均继承了氢氧化物前驱体的球形形 貌,同时所得NCM523 材料具有良好的六方α-NaFeO2 型晶体结构。在电位−pH 图指导下,湿法合成 工艺将一定程度上成为三元正极材料极具前景的制备方法之一。
Similar content being viewed by others
References
PORTHAULT H, LE CRAS F, BADDOUR-HADJEAN R, PEREIRA-RAMOS J P, FRANGER S. One step synthesis of lamellar R-3m LiCoO2 thin films by an electrochemical–hydrothermal method [J]. Electrochimica Acta, 2011, 56(22): 7580–7585. DOI: 10.1016/j.electacta.2011.06.083.
LI Lin, LI Yun-jiao, XU Cang, PAPANGELAKIS V G, CHU Guang, LI Gui-liang, WANG Xuan-yu, KONG Long. E-pH diagrams from 333.15 to 453.15 K for lithium-titanium composite oxides and their synthesis in aqueous solution [J]. Hydrometallurgy, 2014, 142: 131–136. DOI: 10.1016/j. hydromet.2013.11.010.
HOU Pei-yu, ZHANG Hong-zhou, DENG Xiao-long, XU Xi-jin, ZHANG Lian-qi. Stabilizing the electrode/electrolyte interface of LiNi0.8Co0.15Al0.05O2 through tailoring aluminum distribution in microspheres as long-life, high-rate, and safe cathode for lithiumion batteries [J]. ACS Applied Materials & Interfaces, 2017, 9(35): 29643–29653. DOI: 10.1021/ acsami.7b05986.
SONG S W, HAN K S, YOSHIMURA M. Effect of 20-200 °C fabrication temperature on microstructure of hydrothermally prepared LiCoO2 films [J]. Journal of the American Ceramic Society, 2004, 83(11): 2839–2844.
COWAN R L, STAEHLE R W. The thermodynamics and electrode kinetic behavior of nickel in acid solution in the temperature range 25 to 300 °C [J]. Journal of the Electrochemical Society, 1971, 118(4): 557. DOI: 10.1149/1.2408111.
HOU Pei-yu, LI Feng, SUN Yan-yun, LI Hui-qiao, XU Xi-jin, ZHAI Tian-you. Multishell precursors facilitated synthesis of concentration-gradient nickel-rich cathodes for long-life and high-rate lithium-ion batteries [J]. ACS Applied Materials & Interfaces, 2018, 10(29): 24508–24515. DOI: 10.1021/acsami. 8b06286.
LI Feng, KONG Ling-long, SUN Yan-yun, JIN Yong-cheng, HOU Pei-yu. Micron-sized monocrystalline LiNi1/3Co1/3Mn1/3O2 as high-volumetric-energy-density cathode for lithium-ion batteries [J]. Journal of Materials Chemistry A, 2018, 6(26): 12344–12352. DOI: 10.1039/ c8ta03363c.
HOU Pei-yu, YIN Jiang-mei, DING Meng, HUANG Jin-zhao, XU Xi-jin. Surface/interfacial structure and chemistry of high-energy nickel-rich layered oxide cathodes: Advances and perspectives [J]. Small, 2017, 13(45): 1701802. DOI: 10.1002/smll.201701802.
HOU Pei-yu, ZHANG Hong-zhou, ZI Zhong-yue, ZHANG Lian-qi, XU Xi-jin. Core–shell and concentration-gradient cathodes prepared via co-precipitation reaction for advanced lithium-ion batteries [J]. Journal of Materials Chemistry A, 2017, 5(9): 4254–4279. DOI: 10.1039/c6ta10297b.
LEE M H, KANG Y J, MYUNG S T, SUN Y K. Synthetic optimization of Li[Ni1/3Co1/3Mn1/3]O2 via co-precipitation [J]. Electrochimica Acta, 2004, 50(4): 939–948. DOI: 10.1016/ j.electacta.2004.07.038.
LU Hua-quan, ZHOU Hai-tao, SVENSSON A M, FOSSDAL A, SHERIDAN E, LU Shi-gang, VULLUM-BRUER F. High capacity Li[Ni0.8Co0.1Mn0.1O2 synthesized by sol–gel and co-precipitation methods as cathode materials for lithiumion batteries [J]. Solid State Ionics, 2013, 249–250: 105–111. DOI: 10.1016/j.ssi.2013. 07.023.
LIN Yu-kai, LU Chung-hsin. Preparation and electrochemical properties of layer-structured LiNi1/3Co1/3Mn1/3-yAlyO2 [J]. Journal of Power Sources, 2009, 189(1): 353–358. DOI: 10.1016/j.jpowsour.2008.08.072.
WANG Zhi-xing, FANG Hai-sheng, YIN Zhou-lan, LI Xin-hai, GUO Hua-jun, PENG Wen-jie. Synthesis and characterization of high-voltage cathode material LiNi0.5Mn1.5O4 by one-step solid-state reaction [J]. Journal of Central South University of Technology, 2005, 12(1): 54–58. DOI: 10.1007/s11771-005-0371-8.
LI De-cheng, MUTA T, ZHANG Lian-qi, YOSHIO M, NOGUCHI H. Effect of synthesis method on the electrochemical performance of LiNi1/3Mn1/3Co1/3O2 [J]. Journal of Power Sources, 2004, 132(1, 2): 150–155. DOI: 10.1016/j.jpowsour. 2004.01.016.
LIN Bin, WEN Zhao-yin, GU Zhong-hua, HUANG Sha-hua. Morphology and electrochemical performance of Li[Ni1/3Co1/3Mn1/3]O2 cathode material by a slurry spray drying method [J]. Journal of Power Sources, 2008, 175(1): 564–569. DOI: 10.1016/j.jpowsour.2007.09.055.
SHUI Miao, GAO Shan, SHU Jie, ZHENG Wei-dong, XU Dan, CHEN Liang-liang, FENG Lin, REN Yuan-long. LiNi1/3Co1/3Mn1/3O2 cathode materials for LIB prepared by spray pyrolysis. II. Li+ diffusion kinetics [J]. Ionics, 2013, 19(1): 47–52. DOI: 10.1007/s11581-012-0723-y.
PENG Qi-ling, ZHOU Hai-hui, HUANG Zhen-hua, CHEN Jin-hua, KUANG Ya-fei. Catalytic graphitization of polyacrylonitrile-based carbon fibers coated with Prussian blue [J]. Journal of Central South University of Technology, 2010, 17(4): 683–687. DOI: 10.1007/s1171-010-0540-2.
MYUNG S T, LEE M H, KOMABA S, KUMAGAI N, SUN Y K. Hydrothermal synthesis of layered Li[Ni1/3Co1/3Mn1/3]O2 as positive electrode material for lithium secondary battery [J]. Electrochimica Acta, 2005, 50(24): 4800–4806. DOI: 10.1016/j.electacta.2005.02.034.
ZHAO Zhong-wei, HUO Guang-sheng. Thermodynamic and kinetic research of Li2O-H2O system [J]. The Chinese Journal of Nonferrous Metals, 2004(8): 2149–2152. DOI: 10.13182/fst85-a24601. (in Chinese)
MAKIMURA Y, OHZUKU T. Lithium insertion material of LiNi1/2Mn1/2O2 for advanced lithium-ion batteries [J]. Journal of Power Sources, 2003, 119–121: 156–160. DOI: 10.1016/ S0378-7753(03)00170-8.
LI Xue-liang, HE Wen-xiang, CHEN Li, GUO Wei, CHEN Jie-jie, XIAO Zheng-hui. Hydrothermal synthesis and electrochemical performance studies of Al2O3-coated LiNi1/3Co1/3Mn1/3O2 for lithiumion batteries [J]. Ionics, 2014, 20(6): 833–840. DOI: 10.1007/s11581-013-1041-8.
WEN Shi-mei, ZHAO Zhong-wei, HUO Guang-sheng. Thermodynamic analysis and potential-pH diagrams of Li-Co-H2O system [J]. Chinese Journal of Power Source, 2005, 29(7): 423–426. (in Chinese)
GUO Chi-hao, ZHAO Zhong-wei. Thermodynamic analysis on Li-Ni-H2O system [J]. Chinese Journal of Power Source, 2005, 29(6): 7–10. DOI: 10.3969/j.issn.1002-087X.2005.06. 010.(in Chinese)
DEAN J A. Lang’s handbook of chemistry [M]. 3rd ed. Singapore: McGRAW-Hill, 1987.
SONG S, HAN K, SASAGAWA I, WATANABE T, YOSHIMURA M. Effect of LiOH concentration change on simultaneous preparation of LiCoO2 film and powder by hydrothermal method [J]. Solid State Ionics, 2000, 135(1-4): 277–281. DOI: 10.1016/S0167-2738(00)00446-X.
CHEN Yong-xiang, LI Yun-jiao, LI Wei, CAO Guo-lin, TANG Shu-yun, SU Qian-ye, DENG Shi-yi, GUO Jia. High-voltage electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material via the synergetic modification of the Zr/Ti elements [J]. Electrochimica Acta, 2018, 281: 48–59. DOI: 10.1016 /j.electacta.2018.05.154.
ZHU Jie, LI Yun-jiao, XUE Long-long, CHEN Yong-xiang, LEI Tong-xing, DENG Shi-yi, CAO Guo-lin. Enhanced electrochemical performance of Li3PO4 modified Li[Ni0.8Co0.1Mn0.1O2 cathode material via lithium- reactive coating [J]. Journal of Alloys and Compounds, 2019, 773: 112–120. DOI: 10.1016/j.jallcom.2018.09.237.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(FA2019015) supported by the Government of Chongzuo, Guangxi Zhuang Autonomous Region, China; Project(AD18281073) supported by Science and Technology Department of Guangxi Zhuang Autonomous Region
Rights and permissions
About this article
Cite this article
Li, Yj., Li, L., Su, Qy. et al. Thermodynamic analysis of Li-Ni-Co-Mn-H2O system and synthesis of LiNi0.5Co0.2Mn0.3O2 composite oxide via aqueous process. J. Cent. South Univ. 26, 2668–2680 (2019). https://doi.org/10.1007/s11771-019-4204-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-019-4204-6