Abstract
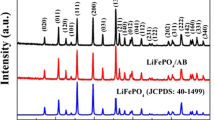
Using low-cost FePO4·2H2O as iron source, Na2FePO4F/C composite is prepared by alcohol-assisted ball milling and solid-state reaction method. The XRD pattern of Na2FePO4F/C composite demonstrates sharp peaks, indicating high crystalline and phase purity. The SEM and TEM images reveal that diameter of the spherical-like Na2FePO4F/C particles ranges from 50 to 300 nm, and HRTEM image shows that the surface of Na2FePO4F/C composite is uniformly coated by carbon layer with a average thickness of about 3.6 nm. The carbon coating constrains the growth of the particles and effectively reduces the agglomeration of nanoparticles. Using lithium metal as anode, the composite delivers a discharge capacities of 102.8, 96.4 and 90.3 mA·h/g at rates of 0.5C, 1C and 2C, respectively. After 100 cycles at 0.5C, a discharge capacity of 98.9 mA·h/g is maintained with capacity retention of 96.2%. The Li+ diffusion coefficient (D) of Na2FePO4F/C composite is calculated as 1.71×10−9 cm2/s. This study reveals that the simple solid state reaction could be a practical and effective synthetic route for the industrial production of Na2FePO4F/C material.
摘要
选用二水合磷酸铁为铁源, 经乙醇辅助球磨和固相反应制备了氟磷酸亚铁钠/碳复合材料。 X- 射线衍射证实产品有高的结晶度和相纯度。 扫描电镜和透射电镜照片显示, 球形氟磷酸亚铁钠粒子的 粒径分布在 50~300 nm 之间; 从高分辨透射电镜图可以看出, 在氟磷酸亚铁钠/碳复合材料的表面包 覆了一层厚度为 3.6 nm 的碳层。碳层的包覆能有效地遏制氟磷酸亚铁钠粒子的长大及粒子的团聚。 以 锂片作为负极组装半电池, 在 0.5C,1C,2C 倍率下,复合材料的放电比容量分别为 102.8,96.4, 90.3 mA·h/g。0.5C 循环 100 次后电池的放电比容量为 98·9 mA·h/g,容量保持率为 96.2%。 从循环伏 安曲线计算得到氟磷酸亚铁钠/碳复合材料的锂离子扩散系数为 1.71×10−9 cm2/s。 显然, 固相法是制备 锂离子电池正极材料用氟磷酸亚铁钠/C 复合材料的有效方法。
Similar content being viewed by others
References
GOODENOUGH J B, PARK K S. The Li-ion rechargeable battery: A perspective [J]. Journal of the American Chemical Society, 2013, 135(4): 1167–1176.
ZHANG Ye, BAI Wen-yu, CHENG Xun-liang, REN Jing, WENG Wei, CHEN Pei-ning, FANG Xin, ZHANG Zhi-tao, PENG Hui-sheng. Flexible and stretchable lithium-ion batteries and supercapacitors based on electrically conducting carbon nanotube fiber springs [J]. Angew Chem Int Ed, 2014, 53: 14564–14568.
DUNN B, KAMATH H, TARASCON J M. Electrical energy storage for the grid: A battery of choices [J]. Science, 2011, 334: 928–935.
LI Wen-liang, NI Er-fu, LI Xin-hai, GUO Hua-jun. Effect of binary conductive additive mixtures on electrochemical performance of polyoxomolybdate as cathode material of lithium ion battery. [J]. Journal of Central South University, 2016, 23: 2506–2512.
LI Hong, WANG Zhao-xiang, CHEN Li-quan, HUANG Xue-jie. Research on advanced materials for Li-ion batteries [J]. Advanced Materials, 2009, 21: 4593–4607.
ANTOLINI E. LiCoO2: Formation, structure, lithium and oxygen nonstoichiometry, electrochemical behaviour and transport properties [J]. Solid State Ionics, 2004, 170(1): 159–171.
LEE M J, LEE S H, OH P, KIM Y S, CHO J. High performance LiMn2O4 cathode materials grown with epitaxial layered nanostructure for Li-ion batteries [J]. Nano Lett, 2014, 14(2): 993–999.
YANG Jin-li, WANG Jia-jun, TANG Yong-ji, WANG Dong-niu, LI Xi-fei, HU Yu-hai, LI Ru-ying, LIANG Guo-xian, SHAM T K, SUN Xue-liang. LiFePO4-graphene as a superior cathode material for rechargeable lithium batteries: Impact of stacked graphene and unfolded graphene [J]. Energy Environ Sci, 2013, 6: 1521–1528.
HOU Hong-shuai, CRAIG E B, JING Ming-jun, ZHANG Yan, JI Xiao-bo. Carbon quantum dots and their derivative 3D porous carbon frameworks for sodium-ion batteries with ultralong cycle life [J]. Advanced Materials, 2015, 27: 7861–7866.
HOU Hong-shuai, SHAO Li-dong, ZHANG Yan, ZOU Guo-qiang, CHEN Jun, JI Xiao-bo. Energy storage: Large-area carbon nanosheets doped with phosphorus: A high-performance anode material for sodium-ion batteries [J]. Advanced Science, 2017, 4: 1600243.
ZHAO Gang-gang, ZHANG Yang, YANG Li, JIANG Yun-ling, ZHANG Yu, HONG Wan-wan, TIAN Ye, ZHAO Hong-bo, HU Jiu-gang, ZHOU Liang, HOU Hong-shuai, JI Xiao-bo, MAI Li-qiang. Nickel chelate derived NiS2 decorated with bifunctional carbon: An efficient strategy to promote sodium storage performance [J]. Advanced Functional Materials, 2018, 28: 1803690.
DENG Ming-xiang, LI Si-jie, HONG Wan-wan, JIANG Yun-ling, XU Wei, SHUAI Hong-lei, ZOU Guo-qiang, HUA Yun-chu, HOU Hong-shuai, WANG Wen-lei, JI Xiao-bo. Octahedral Sb2O3 as high-performance anode for lithium and sodium storage [J]. Materials Chemistry and Physics, 2019, 223: 46–52.
XUE Xia, SUN Dan, ZENG Xian-guang, HUANG Xiao-bing, ZHANG He-he, TANG You-gen, WANG Hai-yan. Two-step carbon modification of NaTi2(PO4)3 with improved sodium storage performance for Na-ion batteries [J]. Journal of Central South University, 2018, 25: 2320–2331.
SHIGETO O, TAKAHASHI Y, KIYABU T, DOI T, YAMAKI J I, NISHIDA T. Layered transition metal oxides as cathodes for sodium secondary battery [J]. Ecs Meeting Abstracts, 2006, 602: 201.
YAMADA Y, DOI T, TANAKA I, OKADA S, YAMAKI J I. Liquid-phase synthesis of highly dispersed NaFeF3 particles and their electrochemical properties for sodium-ion batteries [J]. Journal of Power Sources, 2011, 196: 4837–4841.
LIU Yong-chang, ZHANG Ning, WANG Fan-fan, LIU Xiao-bin, JIAO Li-fang, FAN Li-zhen. Approaching the downsizing limit of maricite NaFePO4 toward highperformance cathode for sodium-ion batteries [J]. Adv Funct Mater, 2018, 28: 1801917–1801925.
RECHAM N, CHOTARD J N, DUPONT L, DJELLAB K, ARMAND M, TARASCON J M, Ionothermal synthesis of sodium-based fluorophosphate cathode materials [J]. Journal of the Electrochemical Society, 2009, 156: A993–A999.
ELLIS B L, MAKAHNOUK W R, MAKIMURA Y, TOGHILL K, NAZAR L F. A multifunctional 3.5 V iron-based phosphate cathode for rechargeable batteries [J]. Nature Materials, 2007, 6: 749–753.
ELLIS B L, MAKAHNOUK W R M, ROWAN- WEETALUKTUK W N, RYAN D H, NAZAR L F. Crystal structure and electrochemical properties of A2MPO4F fluorophosphates (A=Na, Li; M=Fe, Mn, Co, Ni) [J]. Chemistry of Materials, 2010, 22: 1059–1070.
WU Xiao-biao, ZHENG Jian-ming, GONG Zheng-liang, YANG Yong. Sol-gel synthesis and electrochemical properties of fluorophosphates Na2Fe1-xMnxPO4F/C (x=0, 0.1, 0.3, 0.7, 1) composite as cathode materials for Lithium ion battery [J]. Journal of Materials Chemistry, 2011, 21: 18630–18637.
ZHOU Jing-jing, ZHOU Jie-feng, TANG Yuan-hao, BI Yu-jing, WANG Chen-yun, WANG De-yu, SHI Si-qi. Synthesis of Na2FePO4F/C and its electrochemical performance [J]. Ceramics International, 2013, 39: 5379–5385.
SONG Wei-xin, JI Xiao-bo, WU Zheng-ping, ZHU Yi-rong, YAO Yin-peng, HUANGFU K, CHEN Qi-yuan, BANKS C E. Na2FePO4F cathode utilized in hybrid-ion batteries: A mechanistic exploration of ion migration and diffusion capability [J]. Journal of Materials Chemistry A, 2014, 2: 2571.
ELLIS B L, LEE K T, NAZAR L F. Positive electrode materials for Li-ion and Li-batteries [J]. Chemistry of Materials, 2010, 22: 691–714.
BARKER J, SAIDI M Y, SWOYER J L. A sodium-ion cell based on the fluorophosphate compound NaVPO4F [J]. Electrochemical and Solid-State Letters, 2003, 6: A1–A4.
SONG Wei-xin, LIU Su-qin. A sodium vanadium threefluorophosphate cathode for rechargeable batteries synthesized by carbothermal reduction [J]. Solid State Sciences, 2013, 15: 1–6.
KAWABE Y, YABUUCHI N, KAJIYAMA M, FUKUHARA N, INAMASU T, OKUYAMA R, NAKAI I, KOMABA S. Synthesis and electrode performance of carbon coated Na2FePO4F for rechargeable Na batteries [J]. Electrochemistry Communications, 2011, 13: 1225–1228.
BRISBOIS M, CAES S, SOUGRATI M T, VERTRUYEN B, SCHRIJNEMAKERS A, CLOOTS R, ESHRAGHI N, HERMANN R P, MAHMOUD A, BOSCHINI F. Na2FePO4F/multi-walled carbon nanotubes for lithium-ion batteries: Operando Mössbauer study of spray-dried composites [J]. Solar Energy Materials and Solar Cells, 2016, 148: 67–72.
KABALOV Y K, SIMONOV M A, BELOV N V. The crystal structure of sodium iron orthophosphate Na2Fe(PO4)(OH) [J]. Doklady Akademii Nauk SSSR, 1974, 215: 850–853.
SWAFFORD S H, HOLT E M. New synthetic approaches to monophosphate fluoride ceramics: Synthesis and structural characterization of Na2Mg(PO4)F and Sr5(PO4)3F [J]. Solid State Sciences, 2002, 4: 807–812.
SANZ F, PARADA C, RUIZ- VALERO C. Crystal growth, crystal structure and magnetic properties of disodium cobalt fluorophosphate [J]. Journal of Materials Chemistry, 2001, 11: 208–211.
AVDEEV M, LING C D, TAN T T, LI S, OYAMA G, YAMADA A, BARPANDA P. Magnetic structure and properties of the rechargeable battery insertion compound Na2FePO4F [J]. Inorganic Chemistry, 2014, 53: 682–684.
TRIPATHI R, WOOD S M, ISLAM M S, NAZAR L F. Na-ion mobility in layered Na2FePO4F and olivine Na[Fe,Mn]PO4 [J]. Energy & Environmental Science, 2013, 6: 2257–2264.
CUI Dong-ming, CHEN Sha-sha, HAN Chang, AI Changchun, YUAN Liang-jie. Carbothermal reduction synthesis of carbon coated Na2FePO4F for lithium ion batteries [J]. Journal of Power Sources, 2016, 301: 87–92.
BRISBOIS M, KRINS N, HERMANN R P, SCHRIJNEMAKERS A, CLOOTS R, VERTRUYEN B, BOSCHINI F. Spray-drying synthesis of Na2FePO4F/carbon powders for lithium-ion batteries [J]. Materials Letters, 2014, 130: 263–266.
SONG Wei-xin, JI Xiao-bo, WU Zheng-ping, ZHU Yi-rong, LI Fang-qian, YAO Yin-peng, BANKS C E. Multifunctional dual Na3V2(PO4)2F3 cathode for both lithium-ion and sodium-ion batteries [J]. RSC Advances, 2014, 4: 11375–11383.
RUI X H, DING N, LIU J, LI C, CHEN C H. Analysis of the chemical diffusion coefficient of Lithium ions in Li3V2(PO4)3 cathode material [J]. Electrochimi Acta, 2010, 55: 2384–2390.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Projects(51472211, 51502256) supported by the National Natural Science Foundation of China; Projects(2016GK4005, 2016GK4030) supported by the Strategic New Industry of Hunan Province, China; Project(13C925) supported by the Research Foundation of Education Bureau of Hunan Province, China
Rights and permissions
About this article
Cite this article
Hu, H., Wang, Y., Huang, Y. et al. Na2FePO4F/C composite synthesized via a simple solid state route for lithium-ion batteries. J. Cent. South Univ. 26, 1521–1529 (2019). https://doi.org/10.1007/s11771-019-4108-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-019-4108-5
Keywords
- lithium-ion batteries
- Na2FePO4F/C composite
- alcohol-assisted ball milling
- solid state reaction
- spherical-like particles