Abstract
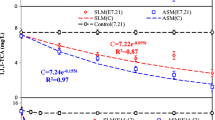
Ethylthionocarbamates (ETC), which is the most widely used as collectors in the flotation of sulfide, is known to cause serious pollution to soil and groundwater. The potential biodegradation of ETC was evaluated by applying a mixed culture of iron-reducing bacteria (IRB) enriched from tailings dam sediments. The results showed that ETC can be degraded by IRB coupled to Fe(III) reduction, both of which can be increased in the presence of anthraquinone-2,6-disulfonate (AQDS). Moreover, Fe(III)-EDTA was found to be a more favorable terminal electron acceptor compared to α-Fe2O3, e.g., within 30 d, 72% of ETC was degraded when α-Fe2O3+AQDS was applied, while it is 82.67% when Fe(III)-EDTA+AQDS is added. The dynamic models indicated that the kETC degradation was decreased in the order of Fe(III)-EDTA+AQDS>α-Fe2O3+AQDS>Fe(III)-EDTA>α-Fe2O3, with the corresponding maximum biodegradation rates being 2.6, 2.45, 2.4 and 2.0 mg/(L.d), respectively, and positive parallel correlations could be observed between kFe(III) and kETC. These findings demonstrate that IRB has a good application prospect in flotation wastewater.
摘要
乙硫氨酯是一种广泛使用的硫化矿捕收剂, 其大量使用给土壤和水体造成了严重的污染。本文 研究了尾矿库底泥沉积物中的异化铁还原混合菌群对乙硫氨酯的降解能力。结果表明: 异化铁还原混 合菌群可以有效地降解乙硫氨酯, 并耦联着铁的还原, 蒽醌-2,6-二磺酸钠的加入可以有效提高乙硫氨 酯的降解速率和铁的还原速率。相对赤铁矿而言, EDTA 络合铁是更好的电子受体, 例如, 在异化铁 还原混合菌群降解乙硫氨酯的过程中, 加入赤铁矿和蒽醌-2,6-二磺酸钠时, 30 d 的乙硫氨酯的去除率 为72%, 而加入EDTA 络合铁和蒽醌-2,6-二磺酸钠时, 30 d 的去除率为82.67%。在加入EDTA 络合 铁和蒽醌-2,6-二磺酸钠、赤铁矿和蒽醌-2,6-二磺酸钠、EDTA 络合铁和赤铁矿条件下, 乙硫氨酯的最 大生物降解速率分别为2.6, 2.45, 2.4 和2.0 mg/(L.d), 铁的还原速率和乙硫氨酯的降解速率常数呈现 很好的正相关性。研究表明异化铁还原菌在浮选废水处理方面具有良好的应用前景。
Similar content being viewed by others
References
CHEN Shao, DU Dong, WU Yi, XING Rui. Degradation of ethylthionocarbamate by activated sludge coupled with interior microelectrolysis [J]. Desalination and Water Treatment, 2016, 57(45): 21437–21443. DOI: 10.1080/19443994.2015.1119743.
CHEN Shao, DU Dong. Degradation of n-butyl xanthate using fly ash as heterogeneous Fentonlike catalyst [J]. Journal of Central South University, 2014, 21(4): 1448–1452. DOI: 10.1007/s11771-014-2084-3.
CUI Kui, HE Yue, JIN Sheng. Enhanced UV-visible response of bismuth subcarbonate nanowires for degradation of xanthate and photocatalytic reaction mechanism [J]. Chemosphere, 2016, 149: 245–253. DOI: 10.1016/j.chemosphere.2016.01.111.
CHEN Xing, HU Yue, PENG Hong, CAO Xue. Degradation of ethyl xanthate in flotation residues by hydrogen peroxide [J]. Journal of Central South University, 2015, 22(2): 495–501. DOI: 10.1007/s11771-015-2548-0.
ESTHER J, SUKLA L B, PRADHAN N, PANDA S. Fe (III) reduction strategies of dissimilatory iron reducing bacteria [J]. Korean Journal of Chemical Engineering, 2015, 32(1): 1–14. DOI: 10.1007/s11814-014-0286-x.
PENG Lai, LIU Yi, GAO Shu, DAI Xiao, NI Bing-Jie. Assessing chromate reduction by dissimilatory iron reducing bacteria using mathematical modeling [J]. Chemosphere, 2015, 139: 334–339. DOI: 10.1016/j.chemosphere.2015.06.090.
LOVLEY D R, ANDERSON R T. Influence of dissimilatory metal reduction on fate of organic and metal contaminants in the subsurface [J]. Hydrogeology Journal, 2000, 8(1): 77–88. https://doi.org/works.bepress.com/derek_lovley/231.
BOND D R, HOLMES D E, TENDER L M, LOVLEY D R. Electrode-reducing microorganisms that harvest energy from marine sediments [J]. Science, 2002, 295: 483–485. DOI: 10.1126/science.1066771.
WILKINS M J, LIVENS F R, VAUGHAN D J, LLOYD J R. The impact of Fe(III)-reducing bacteria on uranium mobility [J]. Biogeochemistry, 2006, 78(2): 125–150. DOI: 10.1007/s10533-005-3655-z.
LI Zhi, SUZUKI D, ZHANG Chun, YANG Su, NAN Jun, YOSHIDA N, WANG Ai, KATAYAMA A. Anaerobic 4-chlorophenol mineralization in an enriched culture under iron-reducing conditions [J]. Journal of Bioscience and Bioengineering, 2014, 118(5): 529–532. DOI: 10.1016/j.jbiosc.2014.04.007.
HE Jiang, QU Dong. Dissimilatory Fe(III) reduction characteristics of paddy soil extract cultures treated with glucose or fatty acids [J]. Journal of Environmental Sciences, 2008, 20(9): 1103–1108.
JIN Xin, WANG Fang, GU Cheng, YANG Xing, KENGARA F O, BIAN Yong, SONG Yang, JIANG Xin. The interactive biotic and abiotic processes of DDT transformation under dissimilatory iron-reducing conditions [J]. Chemosphere, 2015, 138: 18–24. DOI: 10.1016/j. chemosphere.2015.05.020.
DOU Jun, LIU Xiang, DING Ai. Anaerobic degradation of naphthalene by the mixed bacteria under nitrate reducing conditions [J]. Journal of Hazardous Materials, 2009, 165(1–3): 325–331. DOI: 10.1016/j.jhazmat. 2008.10.002.
FENG Chun, YUE Xian, LI Fang, WEI Chao. Bio-current as an indicator for biogenic Fe(II) generation driven by dissimilatory iron reducing bacteria [J]. Biosensors and Bioelectronics, 2013, 39: 51–56. DOI: 10.1016/j.bios.2012.06.037.
LIU Tong, LI Xiao, LI Fang, ZHANG Wei, CHEN Man, ZHOU Shun. Reduction of iron oxides by Klebsiella pneumoniae L17: Kinetics and surface properties [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2011, 379(1–3): 143–150. DOI: 10.1016/j.colsurfa. 2010. 11.061.
XU Yan, HE Yan, FENG Xiao, LIANG Lu, XU Jian, BROOKES P C, WU Jian. Enhanced abiotic and biotic contributions to dechlorination of pentachlorophenol during Fe(III) reduction by an iron-reducing bacterium Clostridium beijerinckii Z [J]. Science of the Total Environment, 2014, 473–474: 215–223. DOI: 10. 1016/j.scitotenv.2013.12.022.
LI Fang, LI Xiao, ZHOU Shun, ZHUANG Li, CAO Fang, HUANG De, XU Wei, LIU Tong, FENG Chun. Enhanced reductive dechlorination of DDT in an anaerobic system of dissimilatory iron-reducing bacteria and iron oxide [J]. Environmental Pollution, 2010, 158: 1733–1740. DOI:10.1016 /j.envpol.2009.11.020.
ZHU Zhen, TAO Liang, LI Fang. 2-nitrophenol reduction promoted by S. putrefaciens 200 and biogenic ferrous iron: The role of different size-fractions of dissolvedorganic matter [J]. Journal of Hazardous Material, 2014, 279(3): 436–443. DOI: 10.1016/j.jhazmat. 2014.07.030.
CAO Fang, LIU Tong, WU Chun, LI Fang, LI Xiao, YU Huan, TONG Hui, CHEN Man. Enhanced biotransformation of DDTs by an iron-and humic-reducing bacteria aeromonashydrophila HS01 upon addition of goethite and anthraquinone-2,6-disulphonicdisodium salt (AQDS) [J]. Journal of Agricultural and Food Chemistry, 2012, 60(45): 11238–11244. DOI: 10.1021/jf303610w.
LUAN Fu, BURGOS W D, XIE Li, ZHOU Qi. Bioreduction of nitrobenzene, naturalorganic matter, and hematite by Shewanella putrefaciens CN32 [J]. Environmental Science & Technology, 2010, 44(1): 184–190. DOI: 10.1021/es901585z@proofing.
NEUMANN A, OLSON T L, SCHERER M M. Spectroscopic evidence for Fe(II)-Fe(III)electron transfer at clay mineral edge and basal sites [J]. Environmental Science & Technology, 2013, 47(13): 6969–6977. DOI: 10.1021/es304744v.
SHEN Wei, CHEN Hong, PAN Shan. Anaerobic biodegradation of 1,4-dioxane by sludge enriched with iron-reducing microorganisms [J]. Bioresource Technology, 2008, 99(7): 2483–2487. DOI: 10.1016/j.biortech.2007. 04.054.
LOVLEY D R, WOODWARD J C. Mechanisms for chelator stimulation of microbial Fe(III)-oxide reduction [J]. Chemical Geology, 1996, 132(1–4): 19–24. DOI: https://doi.org/10.1016/S0009-2541(96)00037-X.
LI Xiao, LIU Liang, LIU Tong, YUAN Tian, ZHANG Wei, LI Fang, ZHOU Shun, LI Yong. Electron transfer capacity dependence of quinone-mediated Fe(III) reduction and current generation by Klebsiella pneumoniae L17 [J]. Chemosphere, 2013, 92(2): 218–224. DOI: 10.1016/j.chemosphere. 2013. 01.098.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(51708561) supported by the National Natural Science Foundation of China; Projects(CZP17097, CZW15037) supported by the Fundamental Research Funds for the Central Universities, China
Rights and permissions
About this article
Cite this article
Chen, Sh., Sun, Y. & Xiong, L. Biodegradation of ethylthionocarbamates by a mixed culture of iron-reducing bacteria enriched from tailings dam sediments. J. Cent. South Univ. 25, 1612–1618 (2018). https://doi.org/10.1007/s11771-018-3853-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-018-3853-1