Abstract
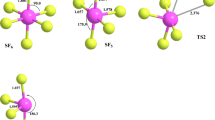
The pyrolysis of 5-HMF was investigated using density functional theory methods at B3LYP/6-31G++(d, p) level. Two possible pyrolytic pathways were proposed and full optimization of the energy gradient for the structures of reactants, products, intermediates and transition states of various reactions was implemented. The standard kinetic parameters in each reaction pathway were calculated and the formation and evolution mechanism of main pyrolysis products were analyzed. Bond dissociation energies calculation results show that the bond dissociation energy of CH3—OH of 5-HMF is the lowest and the order of all kinds of bond dissociation energy is CH3—OH<C—H<CH3OH—Caromatic<CHO—Caromatic<Caromatic—H. In pathway (1), the energy barrier of furfural is 322.8 kJ/mol, the energy barrier of 2-furfuryl alcohol is 375.4 kJ/mol; the energy barrier of furan-2,5-dicarbaldehyde is 496.1 kJ/mol; the energy barrier of 5-methyl furfural is 375.8 kJ/mol, and the energy barrier of 2-methyl furan is 375.8 kJ/mol. In pathway (2), the activation energy required for open-loop with H2O is higher.
Similar content being viewed by others
References
GAO Zheng-wei, WU Zhen, CHEN Wang-qi, KANG Tian-shan. The features and elimination of tar in biomass gasification processes [J]. Guangzhou Chemical Industry, 2015, 43(23): 50–53. (in Chinese)
AHMED I I, GUPTA A K. Kinetics of woodchips char gasification with steam and carbon dioxide [J]. Applied Energy, 2011, 88(5): 1613–1619.
UMEKI K, NAMIOKA T, YOSHIKAWA K. Analysis of an updraft biomass gasifier with high temperature steam using a numerical model [J]. Applied Energy, 2012, 90(1): 38–45.
LIU Hai-li, E Jia-qiang, DENG Yuan-wang, XIE Chang-qing, ZHU Hao. Experimental study on pyrolysis characteristics of the tobacco stem based on the microwave heating method [J]. Applied Thermal Engineering, 2016, 106: 473–479.
LIU Hai-li, E Jia-qiang, MA Xiao-qian, XIE Chang-qing. Influence of microwave drying on the combustion characteristics of food waste [J]. Drying Technology, 2016, 34(12): 1397–1405.
ROBERT J E, THOMAS A M. Molecular characterization of the pyrolysis of biomass 1. Foudamental [J]. Energy & Fuels, 1987, 1(2): 123–137.
ROBERT J E, THOMAS A M. Molecular characterization of the pyrolysis of biomass 2. Applications [J]. Energy & Fuels, 1987, 1(4): 311–319.
LU Q, YANG X, DONG C, ZHANG Z, ZHANG X, ZHU X. Influence of pyrolysis temperature and time on the cellulose fast pyrolysis products: Analytical Py-GC/MS study [J]. Journal of Analytical and Applied Pyrolysis, 2011, 92(2): 430–438.
KATO K. Pyrolysis of cellulose, Part III. Comparative studies of the volatile compounds from pyrolysates of cellulose and its related compounds [J]. Agricultural and Biological Chemistry, 1967, 31: 657–663.
WAN P R, WAN J C, DE J E, RASRENDRA C B, HEERES H J, DE J G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources [J]. Chemical Reviews, 2013, 113(3): 1499–1597.
OSATIASHTIANI A, LEE A F, BROWN D R, MELERO J A, MORALES G, WILSON K. Bifunctional SO4/ZrO2 catalysts for 5-hydroxymethylfufural (5-HMF) production from glucose [J]. Catalysis Science & Technology, 2014, 4(2): 333–342.
OKANO T, QIAO K, BAO Q, YOKOYAMA C. Dehydration of fructose to 5-hydroxymethylfurfural (HMF) in an aqueous acetonitrile biphasic system in the presence of acidic ionic liquids [J]. Applied Catalysis A: General, 2013, 451: 1–5.
JADHAV A H, CHINNAPPAN A, PATIL R H, KOSTJUK S V, KIM H. Green chemical conversion of fructose into 5-hydroxymethylfurfural (HMF) using unsymmetrical dicationic ionic liquids under mild reaction condition [J]. Chemical Engineering Journal, 2014, 243: 92–98.
BINDER J B, RAINES R T. Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals [J]. Journal of the American Chemical Society, 2009, 131(5): 1979–1985.
LU Qiang, LIAO Hang-tao, ZHANG Yang, ZHANG Jun-jiao, DONG Chang-qing. Reaction mechanism of low-temperature fast pyrolysis of fructose to produce 5-hydroxymethyl furfural [J]. Journal of Fuel Chemistry and Technology, 2013, 41(9): 1070–1075. (in Chinese)
HUANG Jin-bao, TONG Hong, LI Wei-min, WU Dan. Quantum chemistry theoretical studies on pyrolysis mechanism of glucopyranose [J]. Chemical Research and Application, 2013, 25(4): 479–483. (in Chinese)
WANG Shu-rong, LUO Zhong-yang. Pyrolysis of biomass components [M]. Beijing: Science Press, 2013. (in Chinese)
SHEN D K, GU S. The mechanism for thermal decomposition of cellulose and its main products [J]. Bioresource Technology, 2009, 100(24): 6496–6504.
HUBER G W, CHHEDA J, BARRETT C B, DUMESIC J A. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates [J]. Science, 2005, 308(27): 1446–1450.
DENG L, LI J, LAI D M, GUO Q X. Catalytic conversion of biomass-derived carbohydrates into-valerolactone without using an external H2 supply [J]. Angewandte Chemie International Edition, 2009, 48(35): 6529–6532.
SHIN E J, NIMLOS M R, EVANS R J. Kinetic analysis of the gas-phase pyrolysis of carbohydrates [J]. Fuel, 2001, 80(12): 1697–1709.
ZHAO Yan, PAN Tao, ZUO Yong, GUO Qing-xiang, FU Yao. Production of aromatic hydrocarbons through catalytic pyrolysis of 5-hydroxymethylfurfural from biomass [J]. Bioresource Technology, 2013, 147: 37–42.
LIAO Yan-fen, GUO Zhen-ge, CAO Ya-wen, MA Xiao-qian, LIN Yan. Analysis of pyrolysis mechanism of 5-hydroxymethyl furfural by using PY-GC-MS and in-situ FT-IR [J]. Journal of South China University of Technology: Natural Science Edition, 2015, 43(6): 15–18. (in Chinese)
ZHANG Fang-pei, CHENG Xin-lu, LIU Zi-jiang, HU Dong, LIU Yong-gang. Density functional studies on the bond dissociation energy and pyrolysis mechanism of propyl nitrate [J]. Chinese Journal of High Pressure Physics, 2005, 19(2): 189–192. (in Chinese)
JI Hui-ling, JIANG Nan, WANG Ji-gang. Theoretical study of the ring-opening mechanisms of constituent units with different numbers of furan rings during the degradation of furfuryl-alcohol resin [J]. Journal of Beijing University of Chemical Technology: Natural Science, 2011, 38(4): 43–46. (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(51276023) supported by the National Natural Science Foundation of China
Rights and permissions
About this article
Cite this article
Chen, B., Shi, Zm., Jiang, Sj. et al. Mechanism studies of 5-HMF pyrolysis by quantum chemistry. J. Cent. South Univ. 24, 2565–2571 (2017). https://doi.org/10.1007/s11771-017-3670-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-017-3670-y