Abstract
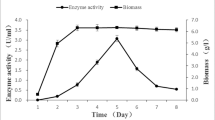
Penicillium simplicissimum was cultured and preserved on the potato dextrose agar (PDA) medium. PDA-RBBR (Remazal Brilliant Blue R) medium was used for the screening of the strains, which is able to produce enzymes. After the mutation process in Penicillium simplicissimum induced by chemical reagent and ultraviolet radiation, a high laccase-producing strains Penicillium simplicissimum was obtained. When 5 mL diethyl sulfate (2%) was mixed along with 5 mL spore suspension for 30 min, chemical mutagenesis reached its best condition. And the optimum conditions of UV mutagenesis were that spore suspension was irradiated for 4 min under 15 W UV lamp at a distance of 30 cm. The highest activity of C5E4 strains was 4.80 U/g over 18% higher than the maximum laccase activity of original microorganism. Five generations of the mutant strains were cultured, and the laccase activity of the strains was measured. The result showed that C5E4 strains can product laccase of the five subcultures stably.
Similar content being viewed by others
References
ALEXANDRE G, ZHULIN I B. Laccases are widespread in bacteria [J]. Trends in Biotechnology, 2000, 18(2): 41–42.
LAI Cui, ZENG Guang-ming, HUANG Dan-lian, ZHAO Mei-hua, WEI Zhen, HUANG Chao, XU Piao, LI Ning-jie, ZHANG Chen, CHEN Ming, LI Xue, LAI Ming-yong, HE Yi-bin. Synthesis of goldcellobiose nanocomposites for colorimetric measurement of cellobiase activity [J]. Spectrochimica Acta Part A, 2014, 132(21): 369–374.
LITTHAUER D. Purification and kinetics of a thermostable laccase from Pycnoporus sanguineus (SCC 108) [J]. Enzyme & Microbial Technology, 2007, 40(4): 563–568.
XU Li-jing, WANG He-xiang, NG T B. A Laccase with HIV-1 reverse transcriptase inhibitory activity from the broth of mycelial culture of the mushroom lentinus tigrinus [J]. Journal of Biomedicine & Biotechnology, 2012(1): 536725–536731.
MUNK L, SITARZ A K, KALYANI D C, MIKKELSEN J D, MEYER A S. Can laccases catalyze bond cleavage in lignin [J]. Biotechnology Advances, 2015, 33(1): 13–24.
ZHOU Yun, DENG Tian-fu, PAN Chen-yuan, CHEN Chun-run, MO Jian-chu. Purification of a laccase from fungus combs in the nest of Odontotermes formosanus [J]. Process Biochemistry, 2010, 45(7): 1052–1056.
BIRHANLI E, YESILADA O. Increased production of laccase by pellets of Funalia trogii ATCC 200800 and Trametes versicolor ATCC 200801 in repeated-batch mode [J]. Enzyme and Microbial Technology, 2006, 39(6): 1286–1293.
FAN Fang-fang, ZHUO Rui, SU Sun, WAN Xia, JIANG Mu-lan, ZHANG Xiao-yu, YANG Yang. Cloning and functional analysis of a new laccase gene from Trametes sp. 48424 which had the high yield of laccase and strong ability for decolorizing different dyes [J]. Bioresource Technology, 2011, 102(3): 3126–3137.
MURUGESAN K, NAM I H, KIM Y M, CHANG Y S. Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture [J]. Enzyme & Microbial Technology, 2007, 40(7): 1662–1672.
DONNA D T, TIWAR R, SAH A K, RAGHUKUMAR C. Enhanced production of laccase by a marine fungus during treatment of colored effluents and synthetic dyes [J]. Enzyme and Microbial Technology, 2006, 38(3, 4): 504–511.
QIU Wei-hua, CHEN Hong-zhang. An alkali-stable enzyme with laccase activity from entophytic fungus and the enzymatic modification of alkali lignin [J]. Bioresource Technology, 2008, 99(13): 5480–5484.
SREBOTNIK E, BOISSON J N. Peroxidation of linoleic acid during the oxidation of phenols by fungal laccase [J]. Enzyme & Microbial Technology, 2005, 36(5, 6): 785–789.
JING De-bin, WANG Jing-hua. Controlling the simultaneous production of laccase and lignin peroxidase from Streptomyces cinnamomensis by medium formulation [J]. Biotechnology for Biofuels, 2012, 5(1): 15–21.
ZENG Guang-ming, YU Hong-Yan, HUANG Hong-Li, HUANG Dan-lian, CHEN Yao-ning, HUANG Guo-he. Laccase activities of a soil fungus Penicillium simplicissimum in relation to lignin degradation [J]. World Journal of Microbiology & Biotechnology, 2006, 22(4): 317–324.
YU Hong-yan, ZENG Guang-ming, HUANG Guo-he, HUANG Dan-lian, CHEN Yao-ning. Lignin degradation by Penicillium simplicissimum [J]. Environmental Science, 2005, 26(2): 167–171. (in Chinese)
SHEN Ying, HU Tian-jue, ZENG Guang-ming, WU Juan-juan, HUANG Chao, LIU Hui. Improving lignin degradation ability of Penicillium simplicissimum by UV induced protoplast mutagenesis [J]. China Environmental Science, 2012, 32(3): 485–491. (in Chinese)
LIU Jian-xiao, ZHOU Wen-Jing, GONG Ji-lai, TANG Lin, ZHANG Yi, YU Hong-yan, WANG Bin, XU Xiang-min, ZENG Guang-ming. An electrochemical sensor for detection of laccase activities from Penicillium simplicissimum in compost based on carbon nanotubes modified glassy carbon electrode [J]. Bioresource Technology, 2008, 99(18): 8748–8751.
XU Ran, TANG Rong-zhi, ZHOU Qi-jun, LI Feng-ting, ZHANG Bing-ru. Enhancement of catalytic activity of immobilized laccase for diclofenac biodegradation by carbon nanotubes [J]. Chemical Engineering Journal, 2015, 262(262): 88–95.
XU Xue-qin, LI Xiao-ming, YANG Qi, LIAO De-xiang, ZENG Guang-ming, ZHANG Yu, LIU Jing-jin. Biosorption of lead and copper ions by Penicillium simplicissimum immobilized on a loofa sponge immobilized biomass [J]. Acta Scientiae Circumstantiae, 2008, 28(1): 95–100. (in Chinese)
RAVELET C, KRIVOBOK S, SAGE L, STEIMAN R. Biodegradation of pyrene by sediment fungi [J]. Chemosphere, 2000, 40(5): 557–563.
TOYAMA T, FURUKAWA T, MAEDA N, INOUE D, SEI K, MORI K, KIKUCHI S, IKE M. Accelerated biodegradation of pyrene and benzo[a]pyrene in the Phragmites australis rhizosphere by bacteria-root exudate interactions [J]. Water Research, 2011, 45(4): 1629–1638.
CHAINEAU C H, MOREL J, DUPONT J, BURY E, OUDOT J. Comparison of the fuel oil biodegradation potential of hydrocarbon-assimilating microorganisms isolated from a temperate agricultural soil [J]. Science of the Total Environment, 1999, 227(2, 3): 237–247.
LIU Hui, HU Tian-jue, ZENG Guang-ming, WU Juan-juang, YANG Chun-ping, ZHANG Ying, SHEN Ying. Power generation using two kinds of phenolic compounds as substrates in MFC [J]. Chinese Journal of Environmental Engineering, 2012, 6(1): 212–217. (in Chinese)
HU Tian-jue, WU Juan-juan, ZENG Guang-ming, LIU Hui, ZHANG Ying, HUANG Dan-lian, YU Bing, SHEN Ying. Research on the degradation effect of contaminants like Phenols and Anilines by Using Penicillium simplicissimum [J]. Journal of Hunan University, 2011, 38(4): 61–65. (in Chinese)
ZHANG Chen, LAI Cui, ZENG Guang-ming, HUANG Dan-lian, YANG Chun-ping, WANG Yang, ZHOU Yao-yu, CHENG Min. Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution [J]. Water Research, 2016, 95: 103–112.
HOU Hong-man, ZHOU Ji-ti, WANG Jing, DU Cui-hong, YAN Bin. Characteristics of laccase from white-rot fungus pleurotus ostreatus strain 3.42 and its decolorization effect on anthraquinone dye [J]. Chemistry & Industry of Forest Products, 2004, 24(1): 48–52. (in Chinese)
MUNOZ C, GUILLEN F, MARTINEZ AT, MARTINEZ M J. Laccase isoenzymes of Pleurotus eryngii: Characterization, catalytic properties, and participation in activation of molecular oxygen and Mn(II) oxidation [J]. Applied & Environmental Microbiology, 1997, 63(6): 2166–2174.
ZHANG Chen, LAI Cui, ZENG Guang-ming, HUANG Dan-lian, TANG Lin, YANG Chun-ping, ZHOU Yao-yu, QIN Lei, CHENG Ming. Nanoporous Au-based chronocoulometric aptasensor for amplified detection of Pb2+ using DNAzyme modified with Au nanoparticles [J]. Biosensors and Bioelectronics, 2016, 81: 61–67.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Foundation item: Projects(51178172, 51308076, 51408206, 51578222) supported by the National Natural Science Foundation of China; Project(113049A) supported by the Ministry of Education of China
Rights and permissions
About this article
Cite this article
Li, X., Li, F., Lai, C. et al. Activities of laccase produced by a strains Penicillium simplicissimum induced by chemical agentia and UV radiation. J. Cent. South Univ. 24, 1953–1958 (2017). https://doi.org/10.1007/s11771-017-3603-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-017-3603-9