Abstract
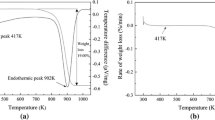
The dissolution property of high-ferrite gibbsitic bauxite and the effect of ferrite content on the dissolution kinetics of gibbsitic bauxites in sodium hydroxide solution under atmospheric pressure from 50 to 90 °C were systematically investigated. The dissolution property of high-ferrite gibbsitic bauxite is increased by increasing the dissolution temperature and the NaOH concentration or decreasing the particle size of bauxite, which is easy to dissolve under atmospheric pressure. The kinetic equations of gibbsitic bauxites with different ferrite contents during the dissolution process at different temperatures for different times were established, and the corresponding activation energies were calculated. The ferrite in the gibbsitic bauxite reduces the dissolution speed and increases the activation energy of dissolution, the diffusion process of which is the rate-controlling step.
Similar content being viewed by others
References
BI Shi-wen, YU Hai-yan. Alumina production technology [M]. Beijing: Chemical Industry Press, 2006: 40–41. (in Chinese)
CERTINI G, WILSON M J, HILLIER S J, FRASER A R, DELBOS E. Mineral weathering in trachydacitic-derived soils and saprolites involving formation of embryonic halloysite and gibbsite at Mt. Amiata, central Italy [J]. Geoderma, 2006, 133(3, 4): 173–190.
MULYANTO B, STOOPS G, VAN R E. Precipitation and dissolution of gibbsite during weathering of andesitic boulders in humid tropical West Java [J]. Geoderma, 1999, 89(3, 4): 287–305.
HERRMANN L, ANONGRAK N, ZAREI M, SCHULER U, SPOHRER K. Factors and processes of gibbsite formation in northern Thailand [J]. Catena, 2007, 71(2): 279–291.
CHEN Jian-guo, LIU Yun-hua, XU Jun-wen. Differences of mineralization of two gibbsitic bauxites in Guangxi province [J]. Earth Science Frontiers, 1999, 6(S): 251–256. (in Chinese)
PEREIRA J A M, SCHWAAB M, DELL'ORO E, PINTO J C, MONTEIRO J L F, HENRIQUES C A. The kinetics of gibbsite dissolution in NaOH [J]. Hydrometallurgy, 2009, 96(1, 2): 6–13.
BAO Li, NGUYEN A V. Developing a physically consistent model for gibbsite leaching kinetics [J]. Hydrometallurgy, 2010, 104(1): 86–98.
ADDAI-MENSAH J, DAWE J, RALSTON J. The dissolution and interactions of gibbsite particles in alkaline media [J]. Developments in Mineral Processing, 2000, 13: C6-1-C6-7.
BAO Li, ZHANG Ting-an, LIU Yan, DOU Zhi-he, LU Guo-zhi, WANG Xiao-min, MA Jia, JIANG Xiao-li. The most probable mechanism function and kinetic parameters of gibbsite dissolution in NaOH [J]. Chinese Journal of Chemical Engineering, 2010, 18(4): 630–634.
YIN Ai-jun, CHEN Qi-yuan, ZHANG Ping-min. Studies on the kinetics of digestion process of synthetic gibbsite by DSC [J]. Chemical Journal of Chinese Universities, 1991, 12(11): 1507–1509. (in Chinese)
LI Chao-qun, ZHANG Ping-min, CHEN Qi-yuan, CHEN Xin-min. Investigation of digestion process kinetics of gibbsite [J]. Nonferrous Metals, 1991, 43(4): 52–55. (in Chinese)
HUA Yi-xin. Introduction to metallurgical process dynamics [M]. Beijing: Metallurgical Industry Press, 2004: 188–198. (in Chinese)
LI Hong-gui. Hydrometallurgy [M]. Changsha: Central South University Press, 2005: 74–79. (in Chinese)
LIU Zhi-xiong, YIN Zhou-lan, CHEN Yi-guang, XIONG Li-zhi. Leaching kinetics of molybdenum from Ni-Mo ore in sulfuric acid solution with sodium peroxodisulfate as oxidant [J]. Journal of Central South University, 2015, 22(3): 874–879.
LIU Zhi-xiong, YIN Zhou-lan, HU Hui-ping, CHEN Qi-yuan. Leaching kinetics of low-grade copper ore with high-alkality gangues in ammonia-ammonium sulphate solution [J]. Journal of Central South University, 2012, 19(1): 77–84.
WEBSTER N A S, LOAN M J, MADSEN I C, KNOTT R B, KIMPTON J A. An investigation of the mechanisms of goethite, hematite and magnetite-seeded Al(OH)3 precipitation from synthetic Bayer liquor [J]. Hydrometallurgy, 2011, 109(1, 2): 72–79.
LIU Hai-bo, CHEN Tian-hu, FROST R L. An overview of the role of goethite surfaces in the environment [J]. Chemosphere, 2014, 103: 1–11.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Projects(51104041, 51174054, 51374065) supported by the National Natural Science Foundation of China; Project(N130402010) supported by the Fundamental Research Funds for the Central Universities of China
Rights and permissions
About this article
Cite this article
Yang, Hb., Pan, Xl., Yu, Hy. et al. Effect of ferrite content on dissolution kinetics of gibbsitic bauxite under atmospheric pressure in NaOH solution. J. Cent. South Univ. 24, 489–495 (2017). https://doi.org/10.1007/s11771-017-3451-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-017-3451-7