Abstract
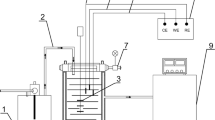
The corrosion behavior of 907 steel under thin electrolyte layer (TEL) has been investigated by means of cathodic polarization curve measurement, electrochemical impedance spectroscopy (EIS) and scanning electron microscopy (SEM). The results show that the cathodic diffusion current density presents the variation trend of initial increase and subsequent decrease with the decrease of TEL thickness, and the maximum deposits at 58 μm. The cotangent-hyperbolic impedance (O) is rationally first introduced to study the diffusion process of the reactants through the corrosion products layer with many permeable holes. The initial corrosion rate of 907 steel under different TEL thickness increases with the decrease of TEL thickness except that of 104 μm, whereas the corrosion rate after long time corrosion can be ranked as 104 μm>402 μm>198 μm>301 μm >bulk solution.
Similar content being viewed by others
References
KATAYAMA H. NODA K, MASUDA H, NAGASAWA M, ITAGAKI M, WATANABE K. Corrosion simulation of carbon steels in atmospheric environment [J]. Corrosion Science, 2005, 47(10): 2599–2606.
HAN Wei, YU Guo-cai, WANG Zhen-yao, WANG Jun. Characterisation of initial atmospheric corrosion carbon steels by field exposure and laboratory simulation [J]. Corrosion Science, 2007, 49(7): 2920–2935.
ABREU C M, CRISTÓBAL M J, NPÓVOA X R, PENA G, PÉREZ M C. Electrochemical behaviour of an AISI 304L stainless steel implanted with nitrogen [J]. Electrochimica Acta, 2008, 53(20): 6000–6007.
XU Jian, WU Xin-qiang, HAN E-H. The evolution of electrochemical behaviour and oxide film properties of 304 stainless steel in high temperature aqueous environment [J]. Electrochimica Acta, 2012, 71: 219–226.
OH S J, COOK D C, TOWNSEND H E. Atmospheric corrosion of different steels in marine, rural and industrial environments [J]. Corrosion Science, 1999, 41(9): 1687–1702.
HOU W, LIANG C. Eight-year atmospheric corrosion exposure of steels in China [J]. Corrosion, 1999, 55(1): 65–73.
MANSFELD F, TSAI S. Laboratory studies of atmospheric corrosion-I. Weight loss and electrochemical measurements [J]. Corrosion Science, 1980, 20(7): 853–872.
MANSFELD F. Atmospheric corrosion rates, time-of-wetness and relative humidity [J]. Materials and Corrosion, 1979, 30(1): 38–42.
STRATMANN M, STRECKEL H. On the atmospheric corrosion of metals which are covered with thin electrolyte layers-I. Verification of the experimental technique [J]. Corrosion Science, 1990, 30(6/7): 681–696.
STRATMANN M, STRECKEL H. On the atmospheric corrosion of metals which are covered with thin electrolyte layers-II. Experimental results [J]. Corrosion Science, 1990, 30(6/7): 697–714.
STRATMANN M, STRECKEL H, KIM K T, CROCKETT S. On the atmospheric corrosion of metals which are covered with thin electrolyte layers-III. The measurement of polarisation curves on metal surfaces which are covered by thin electrolyte layers [J]. Corrosion Science, 1990, 30(6/7): 715–734.
COX A, LYON S B. An electrochemical study of the atmospheric corrosion of mild steel-I. Experimental method [J]. Corrosion Science, 1994, 36(7): 1167–1176.
NISHIKATA A, ICHIHARA Y, TSURU T. An application of electrochemical impedance spectroscopy to atmospheric corrosion study [J]. Corrosion Science, 1995, 37(6): 897–911.
FRANKEL G S, STRATMANN M, ROHWERDER M, MICHALIK A, MAIER B, DORA J, WICINSKI M. Potential control under thin aqueous layers using a Kelvin Probe [J]. Corrosion Science, 2007, 49(4): 2021–2036.
CHENG Ying-liang, ZHANG Zhao, CAO Fa-he, LI Jin-feng, ZHANG Jian-qing, WANG Jian-min, CAO Chu-nan. A study of the corrosion of aluminum alloy 2024-T3 under thin electrolyte layers [J]. Corrosion Science, 2004, 46(7): 1649–1667.
LIU Wen-juan, CAO Fa-he, CHEN An-na, CHANG Lin-rong, ZHANG Jian-qing, CAO Chu-nan. Corrosion behaviour of AM60 magnesium alloys containing Ce or La under thin electrolyte layers. Part 1: Microstructural characterization and electrochemical behaviour [J]. Corrosion Science, 2010, 52(2): 627–638.
LIAO Xiao-ning, CAO Fa-he, ZHENG Li-yun, LIU Wen-juan, CHEN An-na, ZHANG Jian-qing, CAO Chu-nan. Corrosion behaviour of copper under chloride-containing thin electrolyte layer [J]. Corrosion Science, 2011, 53(10): 3289–3298.
ZHANG Tao, CHEN Chong-mu, SHAO Ya-wei, MENG Guo-zhe, WANG Fu-hui, LI Xiao-gang, DONG Chao-fang. Corrosion of pure magnesium under thin electrolyte layers [J]. Electrochimica Acta, 2008, 53(27): 7921–7931.
ZHOU H R, LI X G, MA J, DONG C F, HUANG Y Z. Dependence of the corrosion behavior of aluminum alloy 7075 on the thin electrolyte layers [J]. Materials Science and Engineering B, 2009, 162(1): 1–8.
ZHENG Li-yun, CAO Fa-he, LIU Wen-juan, JIA Bing-li, ZHANG Jian-qing. Corrosion behavior of Pure zinc and its alloy under thin electrolyte layer [J]. Acta Metallurgica Sinica (English Letters), 2010, 23(6): 416–430.
HUANG Hua-liang, DONG Ze-hua, CHEN Zhen-yu, GUO Xing-peng. The effects of Cl− ion concentration and relative humidity on atmospheric corrosion behaviour of PCB-Cu under adsorbed thin electrolyte layer [J]. Corrosion Science, 2011, 53(4): 1230–1236.
SINGH D D N, YADAV S, SAHA J K. Role of climatic conditions on corrosion characteristics of structural steels [J]. Corrosion Science, 2008, 50(1): 93–110.
MA Yuan-tai, LI Ying, WANG Fu-hui. Corrosion of low carbon steel in atmospheric environments of different chloride content [J]. Corrosion Science, 2009, 51(5): 997–1006.
CASTAÑO J G, BOTERO C A, RESTREPO A H, AGUDELO E A, CORREA E, ECHEVERRÍA F. Atmospheric corrosion of carbon steel in Colombia [J]. Corrosion Science, 2010, 52(1): 216–223.
SYED S. Atmospheric corrosion of carbon steel at marine sites in saudi arabia [J]. Materials and Corrosion, 2010, 61(3): 238–244.
WANG Zhi-feng, LIU Jian-rong, WU Li-xin, HAN Rong-dong, SUN Yi-qiang. Study of the corrosion behavior of weathering steels in atmospheric environments [J]. Corrosion Science, 2013, 67: 1–10.
CRUZ R P V, NISHIKATA A, TSURU T. AC impedance monitoring of pitting corrosion of stainless steel under a wet-dry cyclic condition in chloride-dontaining environment [J]. Corrosion Science, 1996, 38(8): 1397–1406.
NISHIKATA A, YAMASHITA Y, KATAYAMA H, TSURU T, USAMI A, TANABE K, MABUCHI H. An electrochemical impedance study on atmospheric corrosion of steels in a cyclic wet-dry condition [J]. Corrosion Science, 1995, 37(12): 2059–2069.
NISHIKATA A, ICHIHARA Y, TSURU T. Electrochemical impedance spectroscopy of metals covered with a thin electrolyte layer [J]. Electrochimica Acta, 1996, 41(7/8): 1057–1062.
NISHIKATA A, ICHIHARA Y, HAYASHI Y, TSURU T. Influence of electrolyte layer thickness and pH on the initial stage of the atmospheric corrosion of iron [J]. Journal of the Electrochemical Society, 1997, 144(4): 1244–1252.
CHUNG K-W, KIM K-B. A study of the effect of concentration build-up of electrolyte on the atmosphericcorrosion of carbonsteel during drying [J]. Corrosion Science, 2000, 42(3): 517–531.
DUBUISSON E, LAVIE P, DALARD F, CAIRE J-P, SZUNERITS S. Study of the atmospheric corrosion of galvanised steel in a micrometric electrolytic droplet [J]. Electrochemistry Communications, 2006, 8(6): 911–915.
HUANG Hua-liang, GUO Xing-peng, ZHANG Guo-an, DONG Ze-hua. The effects of temperature and electric field on atmospheric corrosion behaviour of PCB-Cu under absorbed thin electrolyte layer [J]. Corrosion Science, 2011, 53(5): 1700–1707.
CAO Chu-nan. Estimation of electrochemical kinetic parameters of corrosion processes by weak polarization curve fitting [J]. Journal of Chinese Society for Corrosion and Protection, 1985, 5(3): 155–164.
GUO He-tong, TAN Qi-xian. study of the electrochemistry [M]. Tianjin: Tianjin University Press, 2000: 225. (in Chinese)
CAMPESTRINI P, WESTING E P M, WIT J H W. Influence of surface preparation on performance of chromate conversion coatings on Alclad 2024 aluminium alloy. Part II: EIS investigation [J]. Electrochimica Acta, 2001, 46(17): 2631–2647.
LIU C, BI Q, LEYLAND A, MATTHEWS A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution. Part II: EIS interpretation of corrosion behaviour [J]. Corrosion Science, 2003, 45(6): 1257–1273.
SHAO Hai-bo. Electrochemical behavior and inhibitors of aluminum in alkaline solutions [D]. Hangzhou: Zhejiang University, 2004. (in Chinese)
EI-MAHDY G A, NISHIKATA A, TSURU T. AC impedance study on corrosion of 55%Al-Zn alloy-coated steel under thin electrolyte layers [J]. Corrosion Science, 2000, 42(9): 1509–1521.
LORENZ W J, MANSFELD F. Determination of corrosion rates by electrochemical DC and AC methods [J]. Corrosion Science, 1981, 21(9/10): 647–672.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Projects(21073162, 21273199) supported by the National Natural Science Foundation of China; Project(GCTKF2012013) supported by the Science and Technology Bureau of Jiaxing Municipality and the State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, China
Rights and permissions
About this article
Cite this article
Wang, Hp., Ding, Sc., Zhu, J. et al. Corrosion behavior of 907 steel under thin electrolyte layers of artificial seawater. J. Cent. South Univ. 22, 806–814 (2015). https://doi.org/10.1007/s11771-015-2586-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-015-2586-7