Abstract
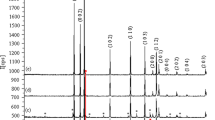
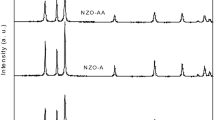
Alkali metal (Li, Na, K) doped ZnO powders were synthesized by solid-state reaction at different calcination temperatures and holding time. Effects of holding time and K sources on the infrared emissivity of ZnO were investigated. The structure and surface morphologies of samples were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). The UV-Vis absorption and infrared emissivities were investigated by a UV-Vis spectrophotometer and an infrared emissometer, respectively. XRD patterns confirm the wurtzite structure of the as prepared samples with single phase. Smooth grain surfaces are detected in all doped ZnO samples, while ZnO:Li and ZnO:Na present the aggregation of grains. The redshifts in the optical band-gap are observed in K-, Na-, and Li-doped ZnO with the values 3.150, 3.144, and 3.142 eV. Due to better crystalline quality, ZnO:K shows a lower emissivity than others. The emissivity of K-doped ZnO decreases to the minimum value (0.804), at 1200 °C and holding 2 h. Compared with KNO3 as K source, K2CO3 doped ZnO has lower emissivities.
Similar content being viewed by others
References
HUANG M H, MAO S, FEICK H, YAN H Q, WU Y Y, KIND H, WEBER E, RUSSO R, YANG P D. Room-temperature ultraviolet nanowire nanolasers [J]. Science, 2001, 292(5523): 1897–1899.
OZGUR U, ALIVOV Y I, LIU C, TEKE A, RESHCHIKOY M A, DOGAN S, AVRUTIN V, CHO S J, MORKOC H. A comprehensive review of ZnO materials and devices [J]. Journal of Applied Physics, 2005, 98(4): 041301–103.
SAITO N, HANEDA H, SEKIGUCHI T, OHASHI N, SAKAGUCHI I, KOUMOTO K. Low-temperature fabrication of light-emitting zinc oxide micropatterns using self-assembled monolayers [J]. Advanced Materials, 2002, 14(6): 418–421.
HSIAO K C, LIAO S C, CHEN Y J. Synthesis, characterization and photocatalytic property of nanostructured Al-doped ZnO powders prepared by spray pyrolysis [J]. Materials Science and Engineering: A, 2007, 447(1): 71–76.
FEI Zhu-ge, YE Zhi-zhen, ZHU Li-ping, LV Jian-guo, ZHAO Bing-hui, HUANG Jing-yun, ZHANG Zheng-hai, LEI Wang, JI Zhen-guo. Electrical and optical properties of Al-N co-doped p-type zinc oxide films [J]. Journal of Crystal Growth, 2004, 268(1): 163–168.
PEI Guang-qing, XIA Chang-tai, WANG Lin-jun, LI Xing, JIAO Xin-bing, XU Jun. Synthesis and characterizations of Al-doped Zn0.95Ni0.05O nanocrystals [J]. Scripta Materialia, 2007, 56(11): 967–970.
COLIS S, BIEBER H, BEGIN-COLIN S, SCHMERBER G, LEUVREY C, DINIA A. Magnetic properties of Co-doped ZnO diluted magnetic semiconductors prepared by low-temperature mechanosynthesis [J]. Chemical Physics Letters, 2006, 422(4): 529–533.
IMAI Y, WATANABE A. Comparison of electronic structures of doped ZnO by various impurity elements calculated by a first-principle pseudopotential method [J]. Journal of Materials Science: Materials in Electronics, 2004, 15(11): 743–749.
DENG R, ZHANG X, ZHANG E, LIANG Y, LIU Z, XU H, HARK S. Planar defects in Sn-doped single-crystal ZnO nanobelts [J]. The Journal of Physical Chemistry C, 2007, 111(35): 13013–13015.
FUJIHARA S, OGAWA Y, KASAI A. Tunable visible photoluminescence from ZnO thin films through Mg-doping and annealing [J]. Chemistry of Materials, 2004, 16(15): 2965–2968.
CHU De-wei, ZENG Yu-ping, JIANG Dong-liang. Synthesis of room-temperature ferromagnetic Co-doped ZnO nanocrystals under a high magnetic field [J]. The Journal of Physical Chemistry C, 2007, 111(16): 5893–5897.
MERON T, MARKOVICH G. Ferromagnetism in colloidal Mn2+-doped ZnO nanocrystals [J]. The Journal of Physical Chemistry B, 2005, 109(43): 20232–20236.
ZHU Dong-mei, LI Kun, LUO Fa, ZHOU Wan-cheng. Preparation and infrared emissivity of ZnO: Al (AZO) thin films [J]. Applied Surface Science, 2009, 255(12): 6145–6148.
WU Xiao-wei, FENG Yu-jie, LIU Yan-kun. Preparation of ZAO powder and investigation on its infrared emissivity [J]. Journal of Harbin Institute Technology (New Series), 2010, 17(4): 588–592.
YAO Yin-hua, CAO Quan-xi. Infrared emissivity of transition elements doped ZnO [J]. Journal of Central South University, 2013, 20: 592–598.
YAO Yin-hua, CAO Quan-xi. Infrared emissivities of Mn, Co co-doped ZnO powders [J]. Chinese Physics B, 2012, 21(12): 124205–6.
JEONG S H, PARK B N, LEE S B, BOO J H. Study on the doping effect of Li-doped ZnO film [J]. Thin Films, 2008, 516(16): 5586–5589.
YANG X P, LU J G, ZHANG H N, CHEN Y, KAN B T, ZHANG J, HUANG J, LU B, ZHANG Y Z, YE Z Z. Preparation and XRD analyses of Na-doped ZnO nanorod arrays based on experiment and theory [J]. Chemical Physics Letters, 2012, 528: 16–20.
GUPTA M K, SINHA N, SINGH B K, KUMAR B. Synthesis of K-doped p-type ZnO nanorods along (100) for ferroelectric and dielectric applications [J]. Materials Letters, 2010, 64(16): 1825–1828.
WANG X S, WU Z C, WEBB J F, LIU Z G. Ferroelectric and dielectric properties of Li-doped ZnO thin films prepared by pulsed laser deposition [J]. Applied Physics A, 2003, 77(3/4): 561–565.
YI J B, LIM C C, XING G Z, FAN H M, VAN L H, HUANG S L, YANG K S, HUANG X L, QIN X B, WANG B Y, WU T, WANG L, ZHANG H T, GAO X Y, LIU T, WEE A T S, FENG Y P, DING J. Ferromagnetism in dilute magnetic semiconductors through defect engineering: Li-Doped ZnO [J]. Physical Review Letters. 2010, 104(13): 137201.
KIM J S, LEE H J, SEOG H J, KIM I W. Dielectric and electrical properties of Li-doped ZnO films [J]. Journal of the Korean Physical Society, 2011, 58(3): 640–644.
RAUCH C, GEHLHOFF W, WANGER M R, MALGUTH E, CALLSEN G, KIRSTE R, SALAMEH B, HOFFMANN A, POLARZ S, AKSU Y, DRIESS M. Lithium related deep and shallow acceptors in Li-doped ZnO nanocrystals [J]. Journal of Applied Physics, 2010, 107(2): 024311–024311-5.
JI Zhen-guo, LIU Fang, HE Hai-yan, HAN Wei-zhi. Characterization and stability of Na-doped p-type ZnO thin films preparation by reactive DC magnetron sputtering [J]. Semiconductor Photonics and Technology. 2009, 15: 139–144.
MOHAMED G A, MOHAMED E, EI-FADL A A. Optical properties and surface morphology of Li-doped ZnO thin films deposited on different substrates by DC magnetron sputtering method [J]. Physica B: Condensed Matter, 2001, 308: 949–953.
KIM S K, KIM S A, LEE C H, LEE H J, JEONG S Y, CHO C R. The structural and optical behaviors of K-doped ZnO/Al2O3(0001) films [J]. Applied Physics Letters. 2004, 85(3): 419–421
CAO Quan-xi, LEI Tian-min, HUANG Yun-xia, LI Gui-fang. Basis of solid physics [M]. Xi’an: Xidian University Press, 2008: 77. (in Chinese)
ZHOU Yu, Ceramic materials [M]. 2nd Eds. Beijing: Science Press, 2004: 12. (in Chinese)
LEITE E R, VARELA J A, LONGO E. Barrier voltage deformation of ZnO varistors by current pulse [J]. Journal of Applied Physics, 1992, 72(1): 147–150.
XU Lin-hua, GU Fang, SU Jing, CHEN Yu-lin, LI Xiang-yin, WANG Xiao-xiong. The evolution behavior of structures and photoluminescence of K-doped ZnO thin films under different annealing temperatures [J]. Journal of Alloys and Compounds, 2011, 509(6): 2942–2947.
LV Jian-guo, CAO Chun-bin, SONG Xue-ping, SUN Zhao-qi. Influence of dopant content on morphology and optical constants of ZnO:Na films by sol-gel method [J]. Journal of Central South University of Technology, 2011, 18(1): 42–47.
SHAN F K, LIU G X, LEE W J, BAE K R, SHIN B C, KIM H S. Structural, electrical, and optical properties of Na-doped ZnO thin films deposited by pulsed laser deposition [J]. Journal of Nanoscience and Nanotechnology, 2008, 8(10): 5203–5207.
GUPTA M K, SINHA N, KUMAR B, p-type K-doped ZnO nanorods for optoelectronic applications [J]. Journal of Applied Physics, 2011, 109(8): 083532–083535.
HU J H, GORDON R G. Atmosphere pressure chemical vapor deposition of gallium doped zinc oxide thin films from diethyil zinc, water, and triethyl gallium [J]. Journal of Applied Physics, 1992, 72(11): 5381–5392.
KIM S K, PAIK U, PARKA J G. Effect of calcination time on the physical properties of synthesized ceria particles for the shallow trench isolation chemical mechanical planarization process [J]. Journal of Ceramic Processing & Research, 2006, 7(1): 53–57.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(JB141405) supported by the Fundamental Research Funds for the Central Universities of China
Rights and permissions
About this article
Cite this article
Li, Hh., Huang, Yx., Li, Zm. et al. Preparation and infrared emissivities of alkali metal doped ZnO powders. J. Cent. South Univ. 21, 3449–3455 (2014). https://doi.org/10.1007/s11771-014-2321-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-014-2321-9