Abstract
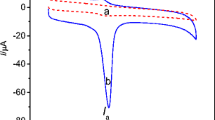
The novel covalently modified glassy carbon electrode with poly(xylitol) was prepared using an electropolymerization technique for the simultaneous determination of uric acid (UA), xanthine (XA) and hypoxanthine (HX). This new electrode presents an excellent electrocatalytic activity towards the oxidation of UA, XA and HX by cyclic voltammetry (CV) method. The oxidation peaks of the three compounds were well defined and had enhanced the peak currents. The separation potentials of the oxidation peak potentials for UA-XA and XA-HX were 380 and 370 mV in CV, respectively. Using differential pulse voltammetry (DPV) method, the calibration curves in the ranges of 5–55, 1.3–75.3 and 4–59 μmol/L were obtained for HX, XA and UA, respectively. The lowest detection limits (S/N=3) were 4.5, 0.75 and 3.75 μmol/L for HX, XA and UA, respectively. The practical application of the modified electrode was demonstrated by the determination of UA, XA, HX in human urine samples.
Similar content being viewed by others
References
PARK G, ADAMS R N, WHITE W R. A rapid accurate electrochemical method for serim uric acid [J]. Anal Lett, 1972, 5: 887–896.
MARTIN C. The Parkinson’s puzzle, new developments in our understanding of Parkinson’s disease have generated a number of promising new treatments for this disabling condition [J]. Chem Br, 1998, 34: 40–42.
BHAGAVAN N V. Medical Biochemistry, [M]. 4th ed. San Diego: Academic Press, 2002: 356–357.
DUTT J S N, CARDOSI M F, DAVIS J. Electrochemical tagging of urate: Developing new redox probes [J]. Analyst, 2003, 128: 811–813.
MATEOS F A, PUIG J G, JIMENEZ M L, FOX I H. Hereditary xanthinuria: Evidence for enhanced hypoxanthine salvage [J]. J Clin Invest, 1987, 79: 847–852.
YAMAMOTO T, MORIWAKI Y, TAKAHASHI S, TSUTSUMI Z, YAMAKITA J, NASAKO Y, NASAKO Y, HIROISHI K, HIGASHINO K. Determination of human plasma xanthine oxidase activity by high performance liquid chromatography [J]. J Chromatogr B, 1996, 681: 395–400.
SHIHABI Z K, HINSDALE M E, BLEYER A J. Xanthine analysis in biological fluids by capillary electrophoresis [J]. J Chromatogr B, 1995, 669: 163–169.
WANG H Y, HUI Q S, XU L X, JIANG J G, SUN Y. Fluorimetric determination of dopamine in pharmaceutical products and urine using ethylene diamine as the fluorigenic reagent [J]. Anal Chim Acta, 2003, 497: 93–99.
HU Y G, LI X X, PANG Z T. Indirect chemiluminescence detection for capillary zone electrophoresis of monoamines and catechol using luminal-K3[Fe(CN)6] system [J]. J Chromatogr A, 2005, 1091: 194–198.
FRAISSE L, BONNET M C, FARCY J P, AGUT C, DERSIGNY D, BAYOLB A. A colorimetric 96-well microtiter plate assay for the determination of urate oxidase activity and its kinetic parameters [J]. Anal Biochem, 2002, 309: 173–179.
DAI X H, FANG X, ZHANG C M, XU R F, XU B. Determination of serum uric acid using high-performance liquid chromatography (HPLC)/isotope dilution mass spectrometry (ID-MS) as a candidate reference method [J]. J Chromatagr B, 2007, 857: 287–295.
TATSUMA T, WATANABE T. Oxidase/peroxidase bilayer-modified electrodes as sensor for lactate, pyruvate, cholesterol and uric acid [J]. Anal Chim Acta, 1991, 242: 85–89.
LIN L Q, CHEN J H, YAO H, CHEN Y Z, ZHENG Y J, LIN X H. Simultaneous determination of dopamine, ascorbic acid and uric acid at poly (evans blue) modified glassy carbon electrode [J]. Bioelectrochemistry 2008, 73: 11–17.
WANG Y, TONG L L. Electrochemical sensor for simultaneous determination of uric acid, xanthine and hypoxanthine based on poly (bromocresol purple) modified glassy carbon electrode [J]. Sensors and Actuators B, 2010, 150: 43–49.
HU G Z, CHEN L, GUO Y, WANG X L, SHAO S J. Selective determination of L-dopa in the presence of uric acid and ascorbic acid at a gold nanoparticle self-assembled carbon nanotube-modified pyrolytic graphite electrode [J]. Electrochimica Acta, 2010, 55: 4711–4716.
BARD A J, Faulkner I R. Electrochemical methods: Fundamentals and applications, [M]. 2nd ed. New York: Wiley, 2000: 87–88.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(201215135) supported by the Natural Science Foundation of Jilin Province, China
Rights and permissions
About this article
Cite this article
Dou, Zy., Cui, Ll. & He, Xq. Electrochimical determination of uric acid, xanthine and hypoxanthine by poly(xylitol) modified glassy carbon electrode. J. Cent. South Univ. 21, 870–876 (2014). https://doi.org/10.1007/s11771-014-2012-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-014-2012-6