Abstract
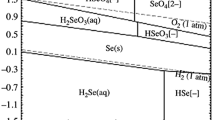
A thermodynamics analysis on the leaching process of selenium residue and discussion on the behaviors of aqueous ionic in the leaching process were carried out. Through thermodynamical calculation, the values of ΔG Θ T and relevant potential expressions were obtained. According to these thermodynamical data, the φ-pH diagrams of Se-H2O system at 298 and 373 K were obtained; Simultaneously, the φ-pH diagrams of SO2-H2O and SO2-Se-H2O systems were drawn at 298 K. With increasing the temperature, the stable regions of HSeO −3 , SeO 2−3 and SeO 2−4 in the φ-pH diagram of Se-H2O system become gradually large, but the limits of pH in the stable region become gradually small. The stability area of reduction precipitation in the SO2-Se-H2O system was finally determined. The results of oxidization leaching experiments of selenium residue indicate that when the mass ratio of selenium residue to sodium chlorate is 2, the concentration of sulfuric acid is 300 g/L and the residue is agitated for 3 h at leaching temperature of 80 °C, the leaching rate of selenium could reach 97.76 %. The experimental results conform the calculated results by φ-pH diagram. The selenium reduction precipitation in oxidization-leaching solution was analyzed under the conditions of acidity of 150 g/L, the sodium sulphite concentration of 35 g/L at the reductive temperature of 23 °C for 120 min. And this demonstrates the thermodynamics analysis.
Similar content being viewed by others
References
WHO Working Group. Selenium [J]. Environmental Health Criteria, 1987, 58(1): 306–311.
ZHOU Ling-zhi. Scare metal extraction metallurgy [M]. Beijing: Metallurgical Industry Press, 2008: 281–316. (in Chinese)
ZHOU Ling-zhi. Handbook of scattered metals [M]. Changsha: Central South University of Technology Press, 1993: 372–374. (in Chinese)
GE Qing-hai, CHEN Hou-xing, XIE Ming-hui. Selenium resources application and the studying situation on separation and abstraction technology [J]. Sichuan Nonferrous Metals, 2005, 9(3): 7–15. (in Chinese)
FENG Cai-xia, LIU Jia-jun, LIU Shen. An outline of selenium resources and its exploitation and utilization [J]. Geology and Resources, 2002, 11(3): 152–156. (in Chinese)
HONG Zuo-min. Selenium resource of China: An outline [J]. Geology of Chemical Minerals, 1997, 19(1): 37–42. (in Chinese)
YANG Chang-jiang, ZHANG Xu, LAN De-jun. Present situation and trend of selenium removal technique from copper anode slime [J]. Sichuan Nonferrous Metals, 2005(1): 22–25. (in Chinese)
ZHOU Ben, ZHAO Chen. Plant practice of process optimizing for treating anode slime [J]. Nonferrous Metals, 2003(1): 26–28. (in Chinese)
YIN Shan-ji, LIU Shi-wu, ZHANG De-jie. Practice of increasing selenium recovery rate from copper anode sludge [J]. China Nonferrous Metallurgy, 2008(3): 28–29. (in Chinese)
ZHOU Ling-zhi, CHEN Shao-chun. Scattered metal extraction metallurgy [M]. Beijing: Metallurgical Industry Press, 2008: 285–292. (in Chinese)
CAI Shi-bing. Separation and recovery of selenium and tellurium from high grade waste material [J]. Hydrometallurgy of China, 2008, 27(1): 35–37. (in Chinese)
MANDRINO D, MILUN M, JENKO M. UHV Se evaporation source: Room-temperature deposition on a clean V (110) surface [J]. Institute of Metals and Technology, 2003, 71(1/2): 267–271.
HUANG Zhan-chao, YANG Bin, DAI Yong-nian. Study on vacuum distillation of selenium residue [J]. Yunnan Metallurgy, 2002, 31(6): 27–29. (in Chinese)
TANG Jia-dao, YANG Xiao-ming. Evolution of crude selenium production process in Yunnan copper Co. Ltd [J]. Yunnan Metallurgy, 2008, 37(4): 31–33. (in Chinese)
WEI Zhi-xian, XU Chun-yan. Solvent extraction of Se (IV) from HCl solution [J]. Journal of North China Institute of Technology, 1996, 17(4): 319–322. (in Chinese)
YANG Wen-bin, WANG Jing-fang, WANG Jian-min. Ion exchange from copper anode slime extract pure selenium [J]. Chinese Journal of Rare Metals, 1989, 13(4): 300–303. (in Chinese)
PEI Liang, HAN Fei, WANG Shuang, WANG Li-ming, ZHANG Lei. New progress of application of ionic liquids in extraction and liquid membrane process [J]. Journal of Analytical Science, 2010, 26(3): 347–352. (in Chinese)
MA Rong-jun. Principle on hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2007: 340–341. (in Chinese)
JIN Zhe-nan, JIANG Kai-xi, WEI Xu-jun, WANG Hai-bei. Potential-pH diagrams of As-S-H2O system at high temperature [J]. Mining and Metals, 1999, 8(4): 45–50. (in Chinese)
YI Xian-wu. An empirical estimation of standard entropy for some complex cations and the E-pH diagrams of As-H2O system at elevated temperature [J]. Journal of Kunming University of Science and Technology, 1982(3): 58–73. (in Chinese)
CRISS C M, COBBLE J W. The thermodynamic properties of high temperature aqueous solutions. IV. Entropies of the ions up to 200 °C and the correspondence principle [J]. Journal of the American Chemical Society, 1964, 86(24): 5385–5390.
ZHONG Zhu-qian, MEI Guang-gui. Application of diagrams of chemical potential in hydrometallurgy and purification of waste water [M]. Changsha: Central South University of Technology Press, 1986: 27–66. (in Chinese)
LIN Chuan-xian, BAI Zheng-hua, ZHANG Zhe-ru. Minerals and related compounds thermodynamics data handbook [M]. Beijing: Science Press, 1985: 31–36. (in Chinese)
LIANG Ying-jiao. Handbook of thermodynamic data of inorganic compounds [M]. Beijing: Metallurgical Industry Press, 2002: 50–89. (in Chinese)
DEAN J A. Lange’s handbook of chemistry [M]. Beijing: Science Press, 2003: 1467–1638.
YANG Xian-wan. Handbook of thermodynamic data in aqueous solutions at high temperature [M]. Beijing: Metallurgical Industry Press, 1983: 523–674. (in Chinese)
FU Cong-yue. Thermodynamic principle and calculation in metallurgical solutions [M]. Beijing: Metallurgical Industry Press, 1989: 154–202. (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(51072233) supported by the National Natural Science Foundation of China
Rights and permissions
About this article
Cite this article
Li, Q., Zhang, B., Min, Xb. et al. Leaching process of selenium residue. J. Cent. South Univ. 19, 2440–2446 (2012). https://doi.org/10.1007/s11771-012-1294-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-012-1294-9