Abstract
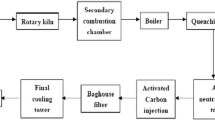
With the addition of urea as an inhibitor, four groups of reducing dioxin emission experiments in sintering pot were conducted. The results show that, adding 0.05%, 0.1% and 0.5% (mass fraction) urea, the emission concentrations of dioxin are 0.287, 0.258 and 0.217 ng-TEQ/m3, respectively. The dioxin emission rates drop substantially compared to 0.777 ng-TEQ/m3 free of urea. With an increase of the urea content, the concentration of SO2 emission reduces sharply. (NH4)2SO4, formed by the reaction of SO2 and NH3, goes into the dust and part of NH3 is released before reaction with the emission of exhaust gas. The NO x emission presents an increasing trend because the reaction of NH3 and O2 at high temperature produces NO x . Based on the consideration of factors such as the effect of reducing dioxin emission, and the chemical composition of exhaust gas, 0.05% is the optimum adding content of urea.
Similar content being viewed by others
References
CHEN Yu-cheng, TSAI Perng-jy, MOU Jin-luh. Determining optimal operation parameters for reducing PCDD/F emissions (I-TEQ values) from the iron ore sintering process by using the Taguchi experimental design [J]. Environ Sci Technol, 2008, 42: 5298–5303.
BOSCOLO M, PADOANO E, TOMMASI S. Identification of possible dioxin emission reduction strategies in pre-existing iron ore sinter plants [J]. Ironmaking and Steelmaking, 2008, 35(2): 146–152.
MENDA N, TAYIBI H, FEMANDO G C, HERMANDEZ A. Minimization methods for emissions generated from sinter strands: A review [J]. Journal of Cleaner Production, 2006, 14: 740–747.
STANMORE B R. The formation of dioxins in combustion systems [J]. Combustion and Frame, 2004, 136(3): 398–427.
PRASHANT S.K, JOAO G. C, CARLOS A M A. Dioxins sources and current remediation technologies-A review [J]. Environment International, 2008, 34: 139–153.
ISHII K, FURUICHI T. Development of bioreactor system for treatment of dioxin-contaminated soil using pseudallescheria boydii [J]. Journal of Hazardous Materials, 2007, 148(3): 693–700.
MASANORI N, KAZUYUKI M, TAKEHIKO S. Factors accelerating dioxin emission from iron ore sintering machines [J]. ISIJ Int, 2009, 49(5): 729–734.
SHUNJI K, YUICHI Y, KAZUOMI W. Investigation on the dioxin emission from a commercial sintering plant [J]. ISIJ Int, 2006, 46(7): 1014–1019.
MASANORI N, YOHZOH H, EIKI K. Observation of behavior of dioxins and some relating elements in iron ore sintering bed by quenching pot test [J]. ISIJ Int, 2005, 45(4): 609–617.
EIKI K, SHUNSUKE K, HIROSHI G, TAICHI M. Reduction in dioxin emissions by the addition of urea as aqueous solution to high-temperature combustion gas [J]. ISIJ Int, 2008, 48(9): 1305–1310.
CHENG He-fa, HU Yua-nan. Curbing dioxin emissions from municipal solid waste incineration in China: Re-thinking about management policies and practices [J]. Environmental Pollution, 2010, 158(9): 2809–2814.
CELINE X, EDWIN D P. Prevention of dioxins de novo formation by ethanolamines [J]. Environ Chem Lett, 2003, 1: 51–56.
FICARELLA A, LAFORGIA D. Numerical simulation of flow-field and dioxins chemistry for incineration plants and experimental investigation [J]. Waste Management, 2000, 20: 27–49.
VALERIE M T, COLIN M M. Relation of chlorine, copper and sulphur to dioxin emission factors [J]. Journal of Hazardous Materials, 2008, 151(1): 164–170.
ANDERSON D R, FISHER R. Sources of dioxins in the United Kingdom: The steel industry and other sources [J]. Chemosphere, 2002, 46: 371–381.
LONG Hong-ming, LI Jia-xin, WANG Ping, GAO Gang, ZHANG Jian. Reaction mechanism of emission of dioxin by addition of urea in iron ore sintering process [J]. The Chinese Journal of Process Engineering, 2010, 10(5): 944–949.
LIAO Ji-yong, BI Xue-gong, XIONG Wei, JIN Yan. Simulation research for sintering waste gas desulfurization [J]. Sintering and Pelletizing, 2006, 31(6): 4–8.
BI Xue-gong, LIAO Ji-yong, XIONG Wei, ZHOU Gou-fan, FENG Zhi-hui. Experimental study of SO2 and NOx removal during sintering process [J]. Journal of Wuhan University of Science and Technology, 2008, 31(5): 449–452.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(50904001) supported by the National Natural Science Foundation of China; Project(2010SQRL032D) supported by Anhui Provincial Key Science Foundation for Outstanding Young Talent, China; Project(TD200909) supported by Program for Innovative Research Team in Anhui University of Technology, China
Rights and permissions
About this article
Cite this article
Long, Hm., Li, Jx. & Wang, P. Influence of dioxin reduction on chemical composition of sintering exhaust gas with adding urea. J. Cent. South Univ. Technol. 19, 1359–1363 (2012). https://doi.org/10.1007/s11771-012-1150-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-012-1150-y