Abstract
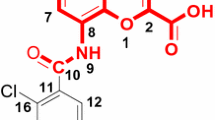
The quantitative structure-activity relationship (QSAR) of 2-alkyl-4-(biphenylylmethoxy) pyridine derivatives was studied. Three different alignment methods were used to get the models of the comparative molecular field analysis (CoMFA), the comparative molecular similarity indices analysis (CoMSIA), and the hologram quantitative structure-activity relationship (HQSAR). The statistical results from the established models show believable predictivity based on the cross-validated value (q 2>0.5) and the non-validated value (r 2>0.9). The analysis on contour maps of CoMFA and CoMSIA models suggests that hydrophobic and hydrogen-bond acceptor fields are important factors that affect the AT1 antagonistic activity of 2-alkyl-4-(biphenylylmethoxy) pyridine derivatives besides the steric and electrostatic fields. The structural modification information from different atom contributions in the HQSAR model is in agreement with that in the 3D-QSAR models.
Similar content being viewed by others
References
FERRARIO C M, STRAWN W B. Role of the rennin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease [J]. The American Journal of Cardiology, 2006, 98: 121–128.
MORSING P, VAUQUELIN G. How can the differences among AT1-receptor antagonists be explained [J]. Cell Biochemistry and Biophysics, 2001, 35: 89–102.
KOSTIS J B, SHELTON B, GOSSELIN G, GOULET C, HOOD W B, KOHN R M, KUBO S H, SCHRON E, WEISS M B, WILLIS III P W, YOUNG G B, PROBSTFIELD J. Adverse effects of enalapril in the studies of left ventricular dysfunction (SOLVD) [J]. American Herat Journal, 1996, 131: 350–355.
TUCCINARDI T, CALDERONE V, RAPPOSELLI S, MARTINELLI A. Proposal of a new binding orientation for non-peptide AT1 antagonists: homology modeling, docking and three dimensional quantitative structure-activity relationship analysis [J]. Journal of Medicinal Chemistry, 2006, 49: 4305–4316.
DARTA P A, DESAI P V, COUTINHO E C. A 3D-QSAR of angiotensin II (AT1) receptor antagonists based on receptor surface analysis [J]. Journal of Chemistry Information and Computer Science, 2004, 44: 210–22.
PARATE A, CHATURVEDI S C. Structural insights for 3H-1, -2, -4 triazolinones as angiotensin II receptor antagonists using QSAR techniques [J]. Medicinal Chemistry Research, 2010, 19(4): 375–391.
TONG W, LOWIS D R, PERKINS R, CHEN Y, WELSH W J, GODDETTE D W, HERITAGE T W, SHEEHAN D M. Evaluation of quantitative structure-activity relationship method for large-scale prediction of chemicals binding to the estrogen receptor [J]. Journal of Chemistry Information and Computer Science, 1998, 38: 669–677.
BRADBURY R H, ALLOTT C P, DENNIS M, GIRDWOOD J A, KENNY P W, MAJOR J S, OLDHAM A A, RATCLIFFE A H, RIVETT J E, ROBERTS D A, ROBINS P J. New nonpeptide angiotensin II receptor antagonists. 3. Synthesis, biological properties, and structure-activity relationships of 2-alkyl-4-(biphenylylmethoxy) pyridine derivatives [J]. Journal of Medicinal Chemistry, 1993, 36: 1245–1254.
TSAI K C, CHEN Y C, HSIAO N W, WANG C L, LIN C L, LEE Y C, LI M, WANG B. A comparison of different electrostatic potentials on prediction accuracy in CoMFA and CoMSIA studies [J]. European Journal of Medicinal Chemistry, 2010, 45: 1544–155.
KAUR K., TALELE T T. Structure-based CoMFA and CoMSIA study of indolinone inhibitor of PDK1 [J]. Journal of Computer-Aided Molecular Design, 2009, 23: 25–26.
ZHU Li-li, XU Xiao-jie. 3D-QSAR analyses of melatonin antagonists [J]. Acta Physico-Chimica Sinica, 2002, 18(12): 1087–1092. (in Chinese)
HAN Xiao-feng, LIU Ying, GAO Ying, LAI Lu-hua. Comparative molecular field analysis of non-peptidic inhibitors of thrombin [J]. Acta Physico-Chimica Sinica, 2003, 61(7): 1136–1139. (in Chinese)
MACCARI R, OTTANÁ R, CURINGA C, VIGORITA MG, RAKOWITZ D, STEINDL T, LANGER T. Structure-activity relationships and molecular modeling of 5-arylidene-2, 4-thiazolidinediones active as aldose reductase inhibitors [J]. Bioorganic & Medicinal Chemistry, 2005, 13: 2809–2823.
KELLOGG G E, SEMUS S F, ABAHAM D J. HINT: A new method of empirical hydrophobic field calculation for CoMFA [J]. Journal of Computer-Aided Molecular Design, 1997, 5: 545–552.
KLEBE G, ABRAHAM U, MIETZNER T. Molecular similarity indices in a comparative analysis (CoMSIA) of drug molecules to correlate and predict their biological activity [J]. Journal of Medicinal Chemistry, 1994, 37: 4130–4146.
BUSH B L, NACHBAR R B. Sample-distance partial least squares: PLS optimized for many variables, with application to CoMFA [J]. Journal of Computer-Aided Molecular Design, 1993, 7: 587–619.
GOLBRAIKH A, TROPSHA A. Beware of q2 [J]. Journal of Molecular Graphics ad Modeling, 2002, 20: 269–276.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(20876180) supported by the National Natural Science Foundation of China
Rights and permissions
About this article
Cite this article
Jiang, Yr., Chen, Yl., Yang, Yy. et al. Quantitative structure-activity relationship of 2-alkyl-4-(biphenylylmethoxy) pyridine derivatives with AT1 receptor antagonistic activity. J. Cent. South Univ. Technol. 19, 1212–1218 (2012). https://doi.org/10.1007/s11771-012-1131-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-012-1131-1