Abstract
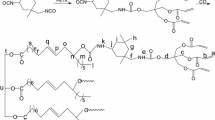
Two kinds of UV curable polyurethane acrylate oligomers (PUPA and PUCA) were synthesized via the addition reaction between isophorone diisocyanate (IPDI) and polyethylene glycol monoacrylate (PEA6) or polycaprolactone modified hydroxyethyl acrylate (PCLA2). The structures of PUPA and PUCA were characterized by Fourier transform infrared spectroscopy (FT-IR), 1H nuclear magnetic resonance (1H NMR), gel permeation chromatography (GPC) and differential scanning calorimeter (DSC), and the thermal stability and dynamic mechanical thermal properties of their cured films were measured by thermogravimetric analysis (TGA) and dynamic mechanical analysis (DMA), respectively. The viscosity of the oligomers and mechanical properties of the cured films were also studied. The results show that both oligomers have narrow molecular weight distribution. The viscosity of PUPA is 2.310 Pa·s at 25 °C, while that of PUCA is up to 3.980 Pa·s. The UV cured PUPA and PUCA films have homogeneous phase structure, and the PUCA film shows higher glass transition temperature and storage modulus. Furthermore, the PUCA film possesses better mechanical properties than PUPA, while the latter shows better alkali resistance.
Similar content being viewed by others
References
CHIANG T H, HSIEH T E. A study of monomer’s effect on adhesion strength of UV-curable resins [J]. International Journal of Adhesion and Adhesives, 2006, 26(7): 520–531.
SRIVASTAVA A, AGARWAL D, MISTRY S, SINGH F. UV curable polyurethane acrylate coatings for metal surfaces [J]. Pigment & Resin Technology, 2008, 37(4): 217–223.
EL-MOLLA M. Synthesis of polyurethane acrylate oligomers as aqueous UV-curable binder for inks of ink jet in textile printing and pigment dyeing [J]. Dyes and Pigments, 2007, 74(2): 371–379.
OPREA S, VLAD S, STANCIU A, MACOVEANU M. Epoxy urethane acrylate [J]. European Polymer Journal, 2000, 36(2): 373–378.
ZHANG Jing, XIAO Pu, SHI Su-qing, NIE Jun. Synthesis and photopolymerization kinetics of multifunctional aromatic urethane acrylates containing tertiary amine group [J]. Polymers for Advanced Technologies, 2009, 20(1): 16–20.
WANG F, HU J Q, TU W P. Study on microstructure of UV-curable polyurethane acrylate films [J]. Progress in Organic Coatings, 2008, 62(3): 245–250.
LIN Y H, LIAO K H, CHOU N K, WANG S S, CHU S H, HSIEH K H. UV-curable low-surface-energy fluorinated poly(urethane-acrylate)s for biomedical applications [J]. European Polymer Journal, 2008, 44(9): 2927–2937.
ZHANG Tong, WU Wen-jian, WANG Xiao-jie, MU Yu-ping. Effect of average functionality on properties of UV-curable waterborne polyurethane-acrylate [J]. Progress in Organic Coatings, 2010, 68(3): 201–207.
LI Zhi-hua, HUANG Yao-peng, REN Dong-yan, ZHENG Zi-qiao. Structural characteristics and properties of polyurethane modified TDE-85/MeTHPA epoxy resin with interpenetrating polymer networks [J]. Journal of Central South University of Technology, 2008, 15(3): 305–308.
ASHA S K, THIRUMAL M, KAVITHA A, PILLAIC K S. Synthesis and curing studies of PPG based telechelic urethane methacrylic macromonomers [J]. European Polymer Journal, 2005, 41(1): 23–33.
HE Yong, ZHOU Meng-bo, WU Bo, JIANG Zhan-lin, NIE Jun. Synthesis and properties of novel polyurethane acrylate containing 3-(2-hydroxyethyl) isocyanurate segment [J]. Progress in Organic Coatings, 2010, 67(3): 264–268.
TAKAHASHI T, WATANABE H, MIYAGAWA N, TAKAHARA S, YAMAOKA T. Application of photopolymer to core-hair type microgels with various hair length [J]. Polymers for Advanced Technologies, 2002, 13(1): 33–39.
KIM C S, KIM B H, KIM K. Synthesis and characterization of polyether urethane acrylate-LiCF3SO3-based polymer electrolytes by UV-curing in lithium batteries [J]. Journal of Power Sources, 1999, 84(1): 12–23.
IGUERB O, BERTRAND P. Graft photopolymerization of polyethylene glycol monoacrylate (PEGA) on poly(methyl methacrylate) (PMMA) films to prevent BSA adsorption [J]. Surface and Interface Analysis, 2008, 40(3/4): 386–390.
TASIC S, BOZIC B, DUNJIC B. Synthesis of new hyperbranched urethane-acrylates and their evaluation in UV-curable coatings [J]. Progress in Organic Coatings, 2004, 51(4): 321–328.
ASHA S K, THIRUMAL M, KAVITHA A, PILLAI C K S. Synthesis and curing studies of PPG based telechelic urethane methacrylic macromonomers [J]. European Polymer Journal, 2005, 41(1): 23–33.
HE Yong, ZHOU Meng-bo, WU Bo, JIANG Zhang-lin, NIE Jun. Synthesis and properties of novel polyurethane acrylate containing 3-(2-hydroxyethyl) isocyanurate segment [J]. Progress in Organic Coatings, 2010, 67(3): 264–268.
CHAN-CHAN L H, SOLIS-CORREA R, VARGAS-CORONADO R F, CERVANTES-UC J M, CAUICH-RODRIGUEZ J V, QUINTANA P, BARTOLO-PÉREZ P. Degradation studies on segmented polyurethanes prepared with HMDI, PCL and different chain extenders [J]. Acta Biomaterialia, 2010, 6(6): 2035–2044.
BARBEAU P H, GERARD J F, MAGNY B, PASCAULT J P. Effect of the diisocyanate on the structure and properties of polyurethane acrylate prepolymers [J]. Journal of Polymer Science Part B: Polymer Physics, 2000, 38(21): 2750–2768.
KIM B, LEE K, JO N. Basic structure-property behavior of UV curable polyurethane acrylates [J]. Journal of Polymer Science Part A: Polymer Chemistry, 2000, 34(11): 2095–2102.
TEY J N, SOUTAR A M, MHAISALKAR S G, YU H, HEW K M. Mechanical properties of UV-curable polyurethane acrylate used in packaging of MEMS devices [J]. Thin Solid Films, 2006, 504(1): 384–390.
YOO H J, LEE Y H, KWON J Y, KIM H D. Comparison of the properties of UV-cured polyurethane acrylates containing different diisocyanates and low molecular weight diols [J]. Fibers and Polymers, 2001, 2(3): 122–128.
ATHAWALE V D, KULKARNI M A. Polyester polyols for waterborne polyurethanes and hybrid dispersions [J]. Progress in Organic Coatings, 2010, 67(1): 44–54.
GITE V V, MAHULIKAR P P, HUNDIWALE D G. Preparation and properties of polyurethane coatings based on acrylic polyols and trimer of isophorone diisocyanate [J]. Progress in Organic Coatings, 2010, 68(4): 307–312.
LU M G, LEE J Y, SHIM M J, KIM S W. Thermal degradation of film cast from aqueous polyurethane dispersions [J]. Journal of Applied Polymer Science, 2002, 85(12): 2552–2558.
SEO J C, JANG E S, SONG J H, CHOI S, KHAN S B, HAN K. Preparation and properties of poly(urethane acrylate) films for ultraviolet-curable coatings [J]. Journal of Applied Polymer Science, 2010, 118(4): 2454–2460.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(2007168303) supported by Guangdong-Hong Kong Technology Cooperation Funding
Rights and permissions
About this article
Cite this article
Liao, F., Zeng, Xr., Li, Hq. et al. Synthesis and properties of UV curable polyurethane acrylates based on two different hydroxyethyl acrylates. J. Cent. South Univ. Technol. 19, 911–917 (2012). https://doi.org/10.1007/s11771-012-1092-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-012-1092-4