Abstract
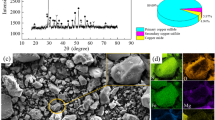
The recovery of nickel from molybdenum leach residue by the process of segregation roasting-sulfuric acid leaching-solvent extraction was investigated. The residue was characterized by microscopic investigations, using X-ray fluorescence spectrometry (XRF) and X-ray diffractometry (XRD) techniques and the residue after segregation roasting was characterized by chemical phase analysis method. A series of experiments were conducted to examine the mass ratio of activated carbon (AC) to the residue, segregation roasting time and temperature, sulfuric acid concentration, liquid-to-solid ratio, leaching time, leaching temperature, addition amount of 30% H2O2, stirring speed (a constant) on the leaching efficiency of nickel. A maximum nickel leaching efficiency of 90.5% is achieved with the mass ratio of AC to the residue of 1:2.5, segregation roasting time of 2 h, segregation roasting temperature of 850 °C, sulfuric acid concentration of 4.5 mol/L, liquid-to-solid ratio of 6:1, leaching time of 5 h, leaching temperature of 80 °C, addition of 30% H2O2 of 0.6 mL for 1 g dry residue. Under these optimized conditions, the average leaching efficiency of nickel is 89.3%. The nickel extraction efficiency in the examined conditions is about 99.6%, and the nickel stripping efficiency in the examined conditions is about 99.2%.
Similar content being viewed by others
References
FAN De-lian, YANG Xiu-zhen, WANG Lian-fang, CHEN Nan-sheng. Petrological and geochemical characteristics of a nickelmolybdenum-multi-element-bearing Lower Cambrian black shale from a certain district in South China [J]. Geochimica, 1973(3): 143–164. (in Chinese)
LI You-yu. Geochemistry of Ni-Mo polymetallic exhalation sediment ore deposit in Northwestern Hunan [J]. Geochimica, 1997, 26(3): 89–96. (in Chinese)
KRIBEK B, SYKOROVA I, PASAVA J, MACHOVIC V. Organic geochemistry and petrology of barren and Mo-Ni-PGE mineralized marine black shales of the Lower Cambrian Niutitang Formation (Southern China) [J]. International Journal of Coal Geology, 2007, 72(3/4): 240–256.
STEINER M, WALLIS E, ERDTMANN B D, ZHAO Yuan-long, YANG Rui-dong. Submarine-hydrothermal exhalative ore layer sin black shales from South China and associated fossils-insights into a Lower Cambrianfacies and bio-evolution [J]. PALA Eos, 2001, 169(3/4): 165–191.
MAO Jing-wen, LEHMANN B, DU An-dao, ZHANG Guang-di, MA Dong-sheng, WANG Yi-tian, ZENG Ming-guo, KERRICH R. Re-Os dating of polymetallic Ni-Mo-PGE-Au mineralization in Lower Cambrian black shales of South China and its geological significance [J]. Economic Geology, 2002, 97(5): 1051–1061.
ORBERGER B, VYMAZALOVÁ A, WAGNER C, FIALIN M, GALLIEN J P, WIRTH R, PAŠAVA J, MONTAGNAC G. Biogenic origin of intergrown Mo-sulphide- and carbonaceous matter in Lower Cambrian black shales (Zunyi Formation, Southern China) [J]. Chem Geol, 2007, 238(3/4): 213–231.
PI Guan-hua, XU Hui, CHEN Bai-zhen, SHI Xi-chang, LI Jun-li. Study on recovering molybdenum from rocky-select Ni-Mo ores [J]. Hunan Nonferrous Metals, 2007, 23(1): 9–12. (in Chinese)
CARON M H. Ammonia leaching of nickel and cobalt ores [J]. Journal of Metals-Transaction AIME, 1950, 88: 67–99.
HOOVER M, HAN K N, FUERSTENAU D W. Reduction roasting of nickel, copper and cobalt from deep-sea manganese nodules [J]. International Journal of Mineral Processing, 1975, 2(2): 173–185.
TITOVA Z P, MAIOROV A D, REZNIK I D, POLOSINA E E, OSIPOVA A S. Extraction of nickel from iron-containing oxidized nickel ores by the segregation method [J]. Tsvetnye Metally, 1975, 48(1): 8–11. (in Russian)
MEHROTRA S P, SRINIVASAN V. Extraction of nickel from an Indian laterite by reduction roasting [J]. Mineral Processing and Extractive Metallurgy, 1994, 103: C97–C104.
ILIC I, KRSTEV B, CEROVIC K, STOPIC S. Study of chlorination of nickel oxide by chlorine and calcium chloride in the presence of active additives [J]. Scandinavian Journal of Metallurgy, 1997, 26(1): 14–19.
LIU Wan-rong, LI Xin-hai, HU Qi-yang, WANG Zhi-xin, GU Ke-zhuan, LI Jin-hui, ZHANG Lian-xin. Pretreatment study on chloridizing segregation and magnetic separation of low-grade nickel laterites [J]. Trans Nonferrous Met Soc China, 2010, 20(s1): s82–s86.
SUDZUKI R. Extraction of nickel from oxidized nickel ores by segregation roasting and magnetic separation [J]. Tsvetnye Metally, 1977(7): 9–12. (in Russian)
PAREKH B K, JEPSEN T L B, GOLDBERGER W M. Segregation roasting and beneficiation of deep sea nodules [J]. Marine Mining, 1988, 7(4): 417–429.
REZNIK I D, TARASOV A V, MAYOROV A D. Mechanism and technology of reduction roasting of oxide nickel ores with subsequent calcine flotation and concentrate leaching [C]// SCHLESINGER M E. EPD Congress 2004. Charlotte City, America: The Minerals, Metals and Materials Society, 2002: 725–737.
KAR B B, DATTA P M, ISRA V N. Thermal study of segregation roasting of pure nickel sulphate [J]. Mineral Processing and Extractive Metallurgy, 2004, 113(2): 118–120.
RUAN H D, FROST R L, KLOPROGGE J T, DUONG L. Infrared spectroscopy of goethite dehydroxylation: II. Effect of aluminium substitution on the behaviour of hydroxyl units [J]. Spectrochim Acta: Part A, 2002, 58(3): 479–491.
VALIX M, CHEUNG W H. Study of phase transformation of laterite ores at high temperature [J]. Miner Eng, 2002, 15(8): 607–612.
LANDERS M, GILKES R J. Dehydroxylation and dissolution of nickeliferous goethite in New Caledonian lateritic Ni ore [J]. Appl Clay Sci, 2007, 35(3/4): 162–172.
McDONALD R G, WHITTINGTON B I. Atmospheric acid leaching of nickel laterites review: Part I. Sulphuric acid technologies [J]. Hydrometallurgy, 2008, 91(1/2/3/4): 35–55.
McDONALD R G, WHITTINGTON B I. Atmospheric acid leaching of nickel laterites review: Part II. Chloride and bio-technologies [J]. Hydrometallurgy, 2008, 91(1/2/3/4): 56–69.
LI Jin-hui, LI Xin-hai, HU Qi-yang, WANG Zhi-xing, ZHOU You-yuan, ZHENG Jun-chao, LIU Wang-rong, LI Ling-jun. Effect of pre-roasting on leaching of laterite [J]. Hydrometally, 2009, 99: 84–88.
ZHAO Si-jia. Recovery and preparation of nickel compounds from residue of metalliferous black shale [D]. Changsha: School of Metallurgical Science and Engineering, Central South University, 2010: 18–30. (in Chinese).
ZHONG Zhu-qian, MEI Guang-gui. Hydrometallurgical processes [M]. Changsha: Central South University of Technology Press, 1988: 83. (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(2007CB613604) supported by the National Basic Research Program of China
Rights and permissions
About this article
Cite this article
Chu, G., Zhao, Sj. & Yang, Tz. Extraction of nickel from molybdenum leaching residue of metalliferous black shale by segregation roasting and acid leaching. J. Cent. South Univ. Technol. 19, 340–346 (2012). https://doi.org/10.1007/s11771-012-1010-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-012-1010-9