Abstract
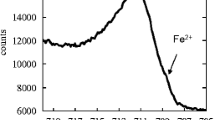
Deep level donor’s ionization behavior of passive film formed on the surface of stainless steel was investigated by Mott-Schottky plots. It is indicated that transformation process of deep level donors’ ionization behavior of passive film on surface of stainless steel can be divided into 4 stages with rising immersion time. At the initial immersion stage (10 min), Fe(II) located in the octahedral sites of the unit cell is not ionized and the deep level does not appear in Mott-Schottky plots. At the second stage (9–38 h), Fe(II) located in the octahedral sites starts to be ionized, which results in deep level donors’ generation and density of deep level donors almost is constant with augmenting immersion time but the thickness of space charge layer is more and more thicker with rising immersion time. At the third stage (48 h–12 d), density of deep level donors rises with increasing immersion time and the thickness of passive films space charge layer decreases. At last stage (above 23 d), both the space charge layer’s thickness and density of deep level donors are no longer changed with increasing immersion time. In the overall immersion stage, the shallow level donors’ density is invariable all the time. The mechanism of deep level donor’s ionization can be the generation of metal vacancies, which results in crystal lattice’s aberration and the aberration energy urges the ionization of Fe(II) in octahedral sites.
Similar content being viewed by others
References
WANG Qiao-ling, HUANG Gui-fang. Effect of sodium nitrite on corrosion of mild steel by using wire beam electrode [J]. Journal of Central South University: Science and Technology, 2008, 39(2): 317–321. (in Chinese)
VIGNAL V, VALOT C, OLTRA R, VERNEAU M, COUDREUSE L. Analogy between the effects of a mechanical and chemical perturbation on the conductivity of passive films [J]. Corrosion Science, 2002, 44(7): 1477–1496.
CARMEZIM M J, SIMÕES A M, MONTEMOR M F, da Cunha Belo M. Capacitance behaviour of passive films on ferritic and austenitic stainless steel [J]. Corrosion Science, 2005, 47(3): 581–591.
MONTEMOR M F, FERREIRA M G S, HAKIKI N E, da Cunha Belo M. Chemical composition and electronic structure of the oxide films formed on 316L stainless steel and nickel based alloys in high temperature aqueous environments [J]. Corrosion Science, 2000, 42(9): 1635–1650.
SIMÕES A M P, FERREIRA M G S, RONDOT B, da Cunha Belo M. Study of passive films formed on AISI 304 stainless steel by impedance measurements and photoelectrochemistry [J]. Journal of the Electrochemical Society, 1990, 137(1): 82–87.
FERREIRA M G S, HAKIKI N E, GOODLET G, FATY S, SIMÕES A M P, da Cunha Belo M. Influence of the temperature of film formation on the electronic structure of oxide films formed on 304 stainless steel [J]. Electrochimica Acta, 2001, 46(24/25): 3767–3776.
HAKIKI N E, MONTEMOR M F, FERREIRA M G S, da Cunha Belo M. Semiconducting properties of thermally grown oxide films on AISI 304 stainless steel [J]. Corrosion Science, 2000, 42(4): 687–702.
ABDULLAH S, SASHA O. Improvement of pitting corrosion resistance of a biomedical grade 316LVM stainless steel by electrochemical modification of the passive film semiconducting properties [J]. Electrochemistry Communications, 2007, 9(1): 76–82.
MONTEMOR M F, SIMÕES A M P, FERREIRA M G S, da Cunha Belo M. The role of Mo in the chemical composition and semiconductive behaviour of oxide films formed on stainless steels [J]. Corrosion Science, 1999, 41(1): 17–34.
CARMEZIM M J, SIMÕES A M, FIGUEIREDO M O, da Cunha Belo M. Electrochemical behaviour of thermally treated Cr-oxide films deposited on stainless steel [J]. Corrosion Science, 2002, 44(3): 451–465.
GOODLET G, FATY S, CARDOSO S, FREITAS P P, SIMÕES A M P, FERREIRA M G S, da Cunha Belo M. The electronic properties of sputtered chromium and iron oxide films [J]. Corrosion Science, 2004, 46(6): 1479–1499.
LEITNER K, SCHLTZE J W, STIMMING U. Photoelectrochemical investigations of passive films on titanium electrodes [J]. Journal of the Electrochemical Society, 1986, 133(8): 1561–1568.
STIMMING U, SCHULTZE J W. Semiconductor model of the passive layer on iron electrodes and its application to electrochemical reaction [J]. Electrochimica Acta, 1979, 24(8): 859–869.
IVERSEN A K. Stainless steels in bipolar plates-Surface resistive properties of corrosion resistant steel grades during current loads [J]. Corrosion Science, 2006, 48(5): 1036–1058.
MORRISON S R. Electrochemistry at semiconductor and oxidized metal electrodes [M]. New York: Plenum, 1980.
SÁNCHEZ M, GREGORI J, ALONSO M C, GARCÍA-JAREŇO J J, VICENTE F. Anodic growth of passive layers on steel rebars in an alkaline medium simulating the concrete pores [J]. Electrochimica Acta, 2006, 52(1): 47–53.
HAMADOU L, KADRI A, BENBRAHIM N. Characterisation of passive films formed on low carbon steel in borate buffer solution (pH=9.2) by electrochemical impedance spectroscopy [J]. Applied Surface Science, 2005, 252(5): 1510–1519.
ALVES V A, BRETT C H A. Characterisation of passive films formed on mild steels in bicarbonate solution by EIS [J]. Electrochimica Acta, 2002, 47(13/14): 2081–2091.
HAKIKI N E, da Cunha Belo M. Electronic structure of passive films formed on molybdenum-containing ferritic stainless steels [J]. Journal of the Electrochemical Society, 1996, 143(10): 3088–3096.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Projects(50571059, 50615024) supported by the National Natural Science Foundation of China; Project(NCET-07-0536) supported by Program for New Century Excellent Talents in University; Project(IRT0739) supported by Program for Innovative Research Team in University
Rights and permissions
About this article
Cite this article
Wang, C., Sheng, Mq., Zhong, Qd. et al. Ionization behavior of deep level donors in passive film formed on surface of stainless steel in 5% salt solution. J. Cent. South Univ. Technol. 17, 295–299 (2010). https://doi.org/10.1007/s11771-010-0045-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-010-0045-z