Abstract
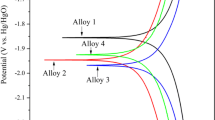
A new anodic material of ternary Pb-0.8%Ag-(0–5.0%)Bi alloy for zinc electrowinning was obtained by doping Bi. The anodic oxygen evolution potential, corrosion rate, surface products after polarization, and microstructures before and after polarization were studied and compared with those of Pb-0.8%Ag anode used in industry. The results show the anodic overpotential decreases with the increase of Bi content in the alloys. When the content of Bi is 1.0% (mass fraction), the anodic overpotential is 40–50 mV lower than that of Pb-0.8%Ag anode. While the corrosion rate decreases and then increases with the increase of Bi content. The Pb-0.8%Ag-0.1%Bi anode has the lowest corrosion rate (0.090 6 mg/(h·cm2). Doping Bi influences the structure of the anodic layer, but does not change the phase. The Pb-0.8%Ag-1.0%Bi anode layer is of a more fine-grained structure compared with Pb-0.8%Ag anode.
Similar content being viewed by others
References
WANG Yan-jun, XIE Gang, YANG Da-jin, LI Yong-jia, XIAO Ting-man. Analysis on decreasing of direct current power consumption in zinc electrowinning [J]. Hydrometallurgy of China, 2005, 24(4): 208–211. (in Chinese)
PFNG Gen-fang. Analyses and optimization exploration of D.C. consumption in zinc electrowinning [J]. Energy Saving of Non-ferrous Metallurgy, 2003, 20(2): 17–20. (in Chinese)
IVANOV I, STEFANOV Y, NONCHEVA Z, PETROVA M, DOBREV T, MIRKOVA L, VERMEERSCH R, DEMAEREL J P. Insoluble anodes used in hydrometallurgy. Part II. Anodic behaviour of Pb and Pb-alloy anodes [J]. Hydrometallurgy, 2000, 57(2): 125–139.
IVANOV I, STEFANOV Y, NONCHEVA Z, PETROVA M, DOBREV T, MIRKOVA L, VERMEERSCH R, DEMAEREL J P. Insoluble anodes used in hydrometallurgy. Part I. Corrosion resistance of Pb and Pb alloy anodes [J]. Hydrometallurgy, 2000, 57(2): 109–124.
STEFANOV Y, DOBREV T. Potentiodynamic and electronmicroscopy investigations of Pb-cobalt alloy coated Pb composite anodes for zinc electrowinning [J]. Transactions of the Institute of Metal Finishing, 2005, 83(6): 296–299.
LI Bao-song, LIN An, GAN Fu-xing. Preparation and electrocatalytic properties of Ti/IrO2-Ta2O5 anodes for oxygen evolution [J]. Trans Nonferrous Met Soc China, 2006, 16(5): 1193–1199.
HU Ji-ming, ZHANG Jian-qing, CAO Chu-nan. Oxygen evolution reaction on IrO2-based DSA type electrodes: Kinetics analysis of Tafel lines and EIS [J]. International Journal of Hydrogen Energy, 2004, 29(8): 791–797.
STEFANOV Y, DOBREV T. Developing and studying the properties of Pb-TiO2 alloy coated Pb composite anodes for zinc electrowinning [J]. Transactions of the Institute of Metal Finishing, 2005, 83(6): 291–295.
ZHONG Shui-ping, LAI Yan-qing, JIANG Liang-xing. Research development of new anode and techniques for zinc electrowinning [J]. Materials Review, 2008, 22(2): 86–89. (in Chinese)
WESSELMARK M, LAGERGREN C, LINDERGH G. Methanol oxidation as anode reaction in zinc electrowinning [J]. Journal of the Electrochemical Society, 2005, 152(11): D201–D207.
JIN Bing-Jie, YANG Xian-wan. Application of gas diffusion anode for zinc hydrometallurgy [J]. Yunnan Metallurgy, 2007, 36(3): 37–39. (in Chinese)
SU Yi, JIN Zuo-mei, DAI Zu-yuan. SO2 anodic reaction kinetics in zinc electrowinning [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(3): 495–498. (in Chinese)
RICE D M. Effects of bismuth on the electrochemical performance of Pb/acid batteries [J]. Journal of Power Sources, 1989, 28(1/2): 69–83.
LI Wei-shan, CHEN Hong-yu, LONG Xue-mei, WU Fan-hua, YAN Jun-hua, ZHANG Cheng-ru. Oxygen evolution reaction on Pb-bismuth alloys in sulfuric acid solution [J]. Journal of Power Sources, 2006, 158(2): 902–907.
HE Qiu-xing TU Wei-ping, HU Jian-qing. Synthesis and characterization of bismuth-doped tin dioxide nanometer powders [J]. Journal of Central South University of Technology, 2006, 13(5): 519–524.
HRUSSANOVA A, MIRKOVA L, DOBREV T. Electrochemical properties of Pb-Sb, Pb-Ca-Sn and Pb-Co3O4 anodes in copper electrowinning [J]. Journal of Applied Electrochemistry, 2002, 32(5): 505–512.
LAITINEN T, SUNDHOLM G, VILHUNEN J K. Comments on sample treatment in the X-ray diffraction analysis of the oxidation products of Pb [J]. Journal of Power Sources, 1990, 32(1): 71–80.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(2007SK2009) supported by the Science and Technology Research Project of Hunan Province, China
Rights and permissions
About this article
Cite this article
Lai, Yq., Zhong, Sp., Jiang, Lx. et al. Effect of doping Bi on oxygen evolution potential and corrosion behavior of Pb-based anode in zinc electrowinning. J. Cent. South Univ. Technol. 16, 236–241 (2009). https://doi.org/10.1007/s11771-009-0040-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-009-0040-4