Abstract
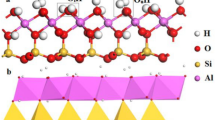
The bulk electronic structure of kaolinite (001) plane was studied with quantum mechanical calculations. The CASTEP parameterization of ultrasoft pseudopotentials without core corrections was used to optimize the structure of kaolinite bulk and slab models. The results show that Fermi energy of kaolinite (001) plane is 3.05 eV, and the band gap is 4.52 eV. The partial density of states (PDOS) of kaolinite (001) plane indicates that Al—O and Si—O bonds on the mineral surface are highly polar. The oxygen atoms of hydroxyl groups in surface layer are capable of forming hydrogen bond with the head group of cationic collectors. The properties of dodecylamine (DDA) cation were also calculated by density function theory (DFT) method at B3LYP/6-31G (d) level for illuminating the flotation processes of kaolinite. Besides the electrostatic attraction, the mechanism between kaolinite and DDA is found to be hydrogen bonds under acidic condition.
Similar content being viewed by others
References
AKIBA E, HAYAKAWA H, HAYASHI S, MIYAWAKI R, TOMURA S, SHIBASAKI Y, IZUMI F, ASANO H, KAMIYAMA T. Structure refinement of synthetic deuterated kaolinite by rietveld analysis using time-of-flight neutron powder diffraction data [J]. Clays and Clay Minerals, 1997, 45(6): 781–788.
BISH D L, VON DREELE R B. Rietveld refinement of non-hydrogen atomic positions in kaolinite [J]. Clays and Clay Minerals, 1989, 37(4): 289–296.
CAO Xue-feng, HU Yue-hua, JIANG Yu-ren. Flotation mechanism of aluminum silicate minerals with N-dodecyl-1, 3-diaminopropane [J]. Chinese Journal of Nonferrous Metals, 2001, 11(4): 693–696. (in Chinese)
LI Hai-pu, HU Yue-hua, JIANG Yu-ren. Interaction mechanism between modified starches and aluminum-silicate minerals [J]. Chinese Journal of Nonferrous Metals, 2001, 11(4): 697–681. (in Chinese)
ZHAO Shi-min, WANG Diang-zuo, HU Yue-hua. Flotation of aluminosilicates using N-(2-aminoethyl)-1-naphthaleneacetamide [J]. Minerals Engineering, 2003, 16(10): 1031–1033.
ZHAO Shi-min, WANG Dian-zuo, HU Yue-hua. A series of aminoamides used for flotation of kaolinite [J]. Journal of University of Science and Technology Beijing, 2005, 12(3): 208–212.
LASAGA A C. Fundamental approaches in describing mineral dissolution and precipitation rates [R]. Washington DC: Mineralogical Society of America, 1995.
SAUER J, UGLIENGO P, GARRONE E, SAUNDERS V R. Theoretical study of van der Waals complexes at surface sites in comparison with the experiment [J]. Chemical Reviews, 1994, 94(7):2095–2160.
COLLINS D R, CATLOW C R. Computer simulations of structures and cohesive properties of micas [J]. American Mineralogist, 1992, 77(11/12): 1172–1181.
BOSENICK A, DOVE M T, MYERS E R, PALIN E J, SAINZ-DIAZ C I, GUITON B S, WARREN M C, CRAIG M S, REDFERN S A T. Computational methods for the study of energies of cation distributions: Applications to cation-ordering phase transitions and solid solutions [J]. Mineralogical Magazine, 2001, 65(2): 193–219.
KUBICKI J D, BLAKE G A, APITZ S E. Ab initio calculations on aluminosilicate Q3 species: Implications for atomic structures of mineral surfaces and dissolution mechanisms of feldspars [J]. American Mineralogist, 1996, 81(7/8): 789–799.
KUBICKI J D, APITZ S E. Molecular cluster models of aluminum oxide and aluminum hydroxide surfaces [J]. American Mineralogist, 1998, 83(9/10): 1054–1066.
SAINZ-DIAZ C I, TIMON V, BOTELLA V, HERNANDEZ-LAGUNA A. Isomorphous substitution effect on the vibration frequencies of hydroxyl groups in molecular cluster models of the clay octahedral sheet [J]. American Mineralogist, 2000, 85(7/8):1038–1045.
SHERMAN D M. Hartree-Fock band structure, equation of state, and pressure-induced hydrogen bonding in brucite, Mg(OH)2 [J]. American Mineralogist, 1991, 76(9/10): 1769–1772.
SMRCOK L, BENCO L. Ab initio periodic Hartree-Fock study of lizardite [J]. American Mineralogist, 1996, 81(11/12): 1405–1412.
WINKLER B, MILMAN V, PAYNE M C. Ab initio total energy studies of minerals using density functional theory and the local density approximation [J]. Mineralogical Magazine, 1995, 59(4):589–596.
WINKLER B, MILMAN V, HYTHA M, PICKARD C, MILMAN V, WARREN M C. Theoretical investigation of bonding in diaspore [J]. European Journal of Mineralogy, 2001, 13(2): 343–349.
BRIDGEMAN C H, BUCKINGHAM A D, SKIPPER N T, PAYNE M C. Ab initio total energy study of uncharged 2:1 clays and their interaction with water [J]. Molecular Physics, 1996, 89(3): 879–888.
EDELBRO R, SANDSTROM A, PAUL J. Full potential calculations on the electron band structures of sphalerite, pyrite and chalcopyrite [J]. Applied Surface Science, 2003, 206(1/4): 300–313.
PAYNE M C, TETER M P, ALLAN D C. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients [J]. Reviews of Modern Physics, 1992, 64(4): 1045–1097.
PERDEW J P, WANG Y. Accurate and simple analytic representation of the electron-gas correlationenergy [J]. Physical Review B, 1992, 45(13): 13244–13249.
WHITE J A, BIRD D M. Implementation of gradientcorrected exchange-correlation potentials in Car-Parrinello total energy calculations [J]. Physical Review B, 1994, 50(21): 4954–4957.
VANDERBILT D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism [J]. Physical Review B, 1990, 41(11): 7892–7895.
GARCIA A, ELSASSER C, ZHU J. Use of gradient-corrected functionals in total energy calculations for solids [J]. Physical Review B, 1992, 46(14): 9829–9832.
ROSSO K M, RUSTAD J R, BYLASKA E J. The Cs/K exchange in muscovite interlayers: An ab initio treatment [J]. Clays and Clay Minerals, 2001, 49(6): 500–513.
BICKMORE B R, ROSSO K M, NAGY K L. Ab intio determination of edge surface structures for dioctahedral 2:1 phyllosilicates: Implications for acid-base reactivity [J]. Clays and Clay Minerals, 2003, 51(4): 359–371.
FRISH M J, TRUCKS G W, SCHLEGEL H B. Gaussian 03, G03RevB.01 [M]. Pennsylvania: Gaussian Inc, 2003.
NEDER R B, BURGHAMMER M, GRASL T. Refinement of the kaolinite structure from single-crystal synchrotron data [J]. Clays and Clay Minerals, 1999, 47(4): 487–494.
HU Yue-hua, LIU Xiao-wen, XU Zheng-he. Role of crystal structure in flotation separation of diaspore from kaolinite, pyrophyllite and illite [J]. Minerals Engineering, 2003, 16(3): 219–227.
NWEMAN A C D. Chemistry of clays and clay minerals [R]. London: Longman Group UK Limited, 1987.
XU Z H, PITT V, LIU Q. Recent advances in reverse flotation of diasporic ores-A Chinese experience [J]. Minerals Engineering, 2004, 17(9/10): 1007–1015.
LEE L T, SOMASUNDARN P. Adsorption of polyacrylamide on oxide minerals [J]. Langmuir, 1989, 5(3): 854–860.
ZHAO Shi-min, WANG Dian-zuo, HU Yue-hua. The flotation behavior of N-(3-aminopropyl)-dodecanamide on three aluminosilicates [J]. Minerals Engineering, 2003, 16(12):1391–1395.
MPOFU P, MENSAH J A, RALSTON J. Investigation of the effect of polymer structure type on flocculation, rheology and dewatering behaviour of kaolinite dispersions [J]. International Journal of Mineral Processing, 2003, 71(1): 247–268.
JIANG Hao, HU Yue-hua, QIN Wen-qing, WANG Yu-hua, WANG Dian-zuo. Mechanism of flotation for diaspore and aluminum-silicate minerals with alkylamine collectors [J]. Chinese Journal of Nonferrous Metals, 2001, 11(4): 688–692. (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(2005CB623701) supported by the Major State Basic Research and Development Program of China; Project(50874118) supported by the National Nature Science Foundation of China; Project(2007B52) supported by the Foundation for the Author of National Excellent Doctoral Dissertation of China
Rights and permissions
About this article
Cite this article
Xia, Ly., Zhong, H., Liu, Gy. et al. Electron bandstructure of kaolinite and its mechanism of flotation using dodecylamine as collector. J. Cent. South Univ. Technol. 16, 73–79 (2009). https://doi.org/10.1007/s11771-009-0012-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-009-0012-8