Abstract
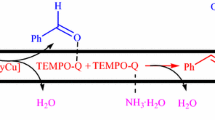
A practical catalytic method to oxidize α-ionone with molecular oxygen using N-hydroxyphthalimide(NHPI) combined with acetylacetone cobatt(II) (Co(acac)2) was developed, and the probable catalytic mechanism was proposed. The influences of the reaction conditions on conversion of α-ionone and the selectivity of the major product (5-keto-α-ionone) were investigated, and the technical parameters for 5-keto-α-ionone were optimized. The results show that the primary product is 5-keto-α-ionone, and by-products include epoxy-α-ionone, as well as rearrangement products 4-keto-β-ionone and epoxy-β-ionone, which are characterized by infrared spectra, proton nuclear magnetic resonance spectra, mass spectra and elemental analysis. The selectivity of 5-keto-α-ionone and the conversion of α-ionone are 55.0% and 97.0%, respectively, when 30%(molar fraction) NHPI, 1.0%(molar fraction) Co(acac)2 and no solvent are employed under O2 pressure of 1.0 MPa and the reaction temperature of 65 °C for 11 h. The procedure shows good reproducibility in the parallel experiments.
Similar content being viewed by others
References
PUNNIYAMURTHY T, VELUSAMY S, IQBAL J. Recent advances in transition metal catalyzed oxidation of organic substrates with molecular oxygen [J]. Chemical Reviews, 2005, 105(6): 2329–2363.
RAJABI F, KARIMI B. Efficient aerobic oxidation of alcohols using a novel combination N-hydroxyphthalimide(NHPI) and a recyclable heterogeneous cobalt complex [J]. Journal of Molecular Catalysis A: Chemical, 2005, 232(1/2): 95–99.
KOGUCHI S, KITAZUME T. Synthetic utilities of ionic liquid-supported NHPI complex [J]. Tetrahedron Lett, 2006, 47(16): 2797–2801.
COSERI S, MENDENHALL G D, INGOLD K U. Mechanisms of reaction of aminoxyl (nitroxide), iminoxyl, and imdoxyl radicals with alkenes and evidence that in the presence of lead tetraacetate, N-Hydroxyphthalimide reacts with alkenes by both radical and nonradical mechanisms [J]. Journal of Organic Chemistry, 2005, 70(12): 4629–4636.
ISHII Y, SAKAGUCHI S. A new strategy for alkane oxidation with O2 using N-hydroxyphthalimide(NHPI) as a radical catalyst [J]. Catalysis Surveys from Japan, 1999, 3(1): 27–35.
IWAHAMA T, HATTA G, SAKAGUCHI S, ISHII Y. Epoxidation of alkenes using alkyl hydroperoxides generated in situ by catalytic autoxidation of hydrocarbons with dioxygen [J]. Chemical Communications, 2000(2): 163–164.
JIANG Si-cui, WU Yang, LI Jun, LIÜ Zhi-ping, LIU Hui. Synthesis of rare fragrance precursor used in tobacco [J]. Fragrance Flavor Cosmetic, 2003, 5(5): 5–6. (in Chinese)
ALEU J, BRENNA E, FUGANTI C, SERRA S. Lipase-mediated synthesis of the enantiomeric forms of 4,5-epoxy-4,5-dihydro-α-ionone and 5, 6-epoxy-5, 6-dihydro-β-ionone: A new direct access to enantiopure (R)-and (S)-α-ionone[J]. Journal of the Chemical Society, Perkin Transactions. 1: Organic and Bio-Organic Chemistry, 1999, 999(3): 271–278.
LIU J, COLMENARES L U, LIU R S M. Fluorinated astaxanthins [J]. Tetrahedron Lett, 1997, 38(49): 8495–8498.
LUO Yi-ming, LIU Chang-hui, TANG Rui-ren, YANG Hua-wu. Synthesis of 4-oxo-β-ionone by oxidation of sodium chlorate [J]. Journal of Central South University: Science and Technology, 2006, 37(3): 521–526. (in Chinese)
PENG Qian-rong, YANG Min, XIE Ru-gang, SONG Guang-fu, LIU Zhong-xiang, WANG Dong-shan, CAI Yuan-qing. A synthesis method of oxo-α-ionone, oxo-β-ionone and their ether and ester derivatives by one step. China: 1817842A [P]. 2006-08-16. (in Chinese)
ZHUO Guang-lan, ZHAO Wei-juan, JIANG Xuan-zhen. A novel catalyst system for the oxidation of toluene to benzoic acid [J]. Chinese Journal of Organic Chemistry, 2004, 24(8): 962–965. (in Chinese)
HIRAI N, SAWATARI N, NAKAMURA N, SAKAGUCHI S, ISHII Y. Oxidation of substituted toluenes with molecular oxygen in the presence of N, N′, N″-trihydroxyisocyanuric acid as a key catalyst [J]. Journal of Organic Chemistry, 2003, 68(17): 6587–6590.
TANG Rui-ren, LIU Chang-hui, LUO Yi-ming, GUO Can-cheng. Studies on the catalytic oxidation of β-ionone to 4-oxo-β-ionone [J]. Journal of Applied Chemistry, 2006, 23(7): 718–723. (in Chinese)
SHELDON R A, ARENDS I W C E. Organocatalytic oxidations mediated by nitroxyl radicals [J]. Advanced Synthesis and Catalysis, 2004, 346(9/10): 1051–1071.
FAN Qian, LI Yao-zhong, CHENG Pu-ming, HU Jia-yuan, LI Xian-jun. Studies on allylic oxidation in cyclohexene [J]. Chemical Research and Application, 2001, 13(5): 557–559. (in Chinese)
ISHII Y, SAKAGUCHI S. Recent progress in aerobic oxidation of hydrocarbons by N-hydroxyimides [J]. Catalysis Today, 2006, 117(1/3): 105–113.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(50573019) supported by the National Natural Science Foundation of China
Rights and permissions
About this article
Cite this article
Tang, Rr., Zhou, Yp., Gong, Nh. et al. Aerobic oxidation behavior of α-ionone catalyzed by N-hydroxyphthalimide combined with acetylacetone cobalt(II). J. Cent. South Univ. Technol. 15, 474–478 (2008). https://doi.org/10.1007/s11771-008-0089-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-008-0089-5