Abstract
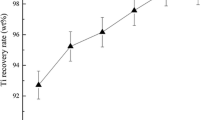
A new technology was developed to recover multiple valuable elements from the spent Al2O3-based catalyst by X-ray phase analysis and exploratory experiments. The experimental results show that in the condition of roasting temperature of 750 °C and roasting time of 30 min, molar ratio of Na2O to Al2O3 of 1.2, the leaching rates of alumina, vanadium and molybdenum in the spent catalyst are 97.2%, 95.8% and 98.9%, respectively. Vanadium and molybdenum in sodium aluminate solution can be recovered by precipitators A and B, and the precipitation rates of vanadium and molybdenum are 94.8% and 92.6%. Al(OH)3 was prepared from sodium aluminate solution in the carbonation decomposition process, and the purity of Al2O3 is 99.9% after calcination, the recovery of alumina reaches 90.6% in the whole process; the Ni-Co concentrate was leached by sulfuric acid, a nickel recovery of 98.2% and cobalt recovery over 98.5% can be obtained under the experimental condition of 30% H2SO4, 80°C, reaction time 4 h, mass ratio of liquid to solid 8, stirring rate 800 r/min.
Similar content being viewed by others
References
Anon L. Refining catalyst demand[J]. Oil and Gas Journal, 2000, 98(41): 64–66.
Rapaport D. Are spent hydrocracking catalysts listed hazardous wastes? [J]. Hydrocarbon Processing, 2000, 79(7): 49–53.
Chang T. Reclamation and landfill processes are alternatives to regeneration[J]. Oil and Gas Journal, 1998, 96(42): 79–84.
Trimm D L. The regeneration or disposal of deactivated heterogeneous catalysts[J]. Applied Catalysis A: General, 2001, 212(1): 153–160.
Marafi M, Stanislaus A. Options and processes for spent catalyst handling and utilization[J]. Journal of Hazardous Materials, 2003, 101(2): 123–132.
LIU Huan-qun. Recovering of spent catalyst in the foreign country[J]. Chinese Resource Comprehensive Utilization, 2000(12): 35–37. (in Chinese)
Toukai K T, Katsuta K K, Toubai H S, et al. Process for recovering valuable metal from waste catalyst. US, 5431892[P]. 1995 - 07 -11.
Veal J T, Andersen K A, Kowaleski R M. Process to recover metals from spent catalyst. US, 6180072 B1 [P]. 2001 - 01 - 30.
Yoo J S. Metal recovery and rejuvenation of metalloaded spent catalysts[J]. Catalysis Today, 1998, 44(1): 27–46.
Mansi A, Monem A. Recovery of nickel oxide from spent catalyst[J]. Waste Management, 2002, 22: 85–90.
Chmielewski A G, Urbanski T S, Migdal W. Separation technologies for metals recovery from industrial wastes[J]. Hydrometallurgy, 1997, 45 (3): 333–344.
Inoue K, Zhang P, Tsuyama H. Separation and recovery of rare metals from spent hydrodesulfurization catalysts by solvent extraction[C] // Third International Symposium on Recycling of Metals and Engineered Materials. Pennsylvania: Minerals, Metals and Materials Society, 1995: 393–404.
Furimsky E. Spent refinery catalysts: environment, safety and utilization[J]. Catalysis Today, 1996, 30(4): 223–236.
Luo L, Miyazaki T, Shibayama A, et al. A novel process for recovery of tungsten and vanadium from a leach solution of tungsten alloy scrap[J]. Minerals Engineering, 2003, 16(7): 665–670.
Radchenko E D, Nefedov B R, Aliev R R. Catalyst of crude oil deep processing[M]. Translated by LI Feng-xiao et al. Beijing: Chinese Petrochemical Industry Press, 1998. (in Chinese)
Soldenhoff K, Hayward N, Wilkins D. Direct solvent extraction of cobalt and nickel from laterite-acid pressure leach liquors[C] // EPD Congress. Pennsylvania: Minerals, Metals and Materials Society, 1998: 153–165.
Cheng C H. Purification of synthetic laterite leach solution by solvent extraction using D2EHPA [J]. Hydrometallurgy, 2000, 56(3): 369–386.
Tsakiridis P E, Agatzini S L. Process for the recovery of cobalt and nickel in the presence of magnesium and calcium from sulphate solutions by Versatic 10 and Cyanex 272[J]. Minerals Engineering, 2004, 17(4): 535–543.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(2003UDBEA00C020) supported by the Collaborative Project of School and Province of Yunnan Province
Rights and permissions
About this article
Cite this article
Feng, Qm., Chen, Y., Shao, Yh. et al. New technique of comprehensive utilization of spent Al2O3-based catalyst. J Cent. South Univ. Technol. 13, 151–155 (2006). https://doi.org/10.1007/s11771-006-0147-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-006-0147-9
Key words
- spent Al2O3-based catalyst
- vanadium
- molybdenum
- comprehensive utilization
- roasting with sodium
- leaching rate