Abstract
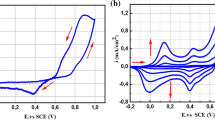
A novel polyaniline-graphite composite film glucose oxidase (PGCF GOD) electrode was developed. The PGCF was synthesized by cyclic voltammetry method in 0.5 mol/L H2SO4 solution containing 1 g/L graphite powder and 0.2 mol/L aniline. The PGCF GOD electrode was prepared by doping GOD into the composite film. The morphology of the PGCF and the response property of the PGCF GOD electrode were investigated by scanning electron microscopy and electrochemical measurement, respectively. The results show that the PGCF has a porous and netty structure and the PGCF GOD electrode has excellent response property such as high sensitivity and short response time. Influences of pH value, temperature, glucose concentration and potential on the response current of the electrode were also discussed. The sensor has a maximum steady-state current density of 357.17 μA/cm2 and an apparent Michaelis-Menten constant of 16.57 mmol/L. The maximum current response of the enzyme electrode occurs under the condition of pH 5.5, 0.8 V and 65 °C.
Similar content being viewed by others
References
Gerard M, Malhotra B D. Application of polyaniline as enzyme based biosensor[J]. Current Applied Physics, 2005, 5(2): 174–177.
Suna T, Nurdan P, Roberto P, et al. Thick film sensors based on laccases from different sources immobilized in polyaniline matrix[J]. Sensors and Actuators B, 2004, 97(1): 132–136.
Ntlatseng G R, Mathebe A M, Emmanuel I I. Electrochemistry and scanning electron microscopy of polyaniline/peroxidase-based biosensor[J]. Talanta, 2004, 64(1): 115–120.
Won J S, You H B. A glucose oxidase electrode based on electropolymerized conducting polymer with polyanion-enzyme conjugated dopant[J]. Analytical Chemistry, 2000, 72(9): 2177–2181.
Umana M, Waller J. Protein-modified electrodes: the glucose oxidase/polypymole system[J]. Analytical Chemistry, 1986, 58 (14): 2979–2983.
Chaubey A, Pande K K, Singh V S, et al. Conducting polyaniline films[J]. Analytical Chimica Acta, 2000, 407 (1/2): 97–103.
Yang Y F, Mu S L. Bioelectrochemical responses of the polyaniline horseradish peroxidase electrodes [J]. Journal of Electroanalytical Chemistry, 1997, 432(1/2): 71–78.
Croissant M J, Napporn T, Leger J M, et al. Electrocatalytic oxidation of hydrogen at platinum-modified polyaniline electrodes[J]. Electrochimica Acta, 1998, 43(16/17): 2447–2457.
Evtugyn G, Mingaleva A, Budnikov H, et al. Affinity biosensors based on disposable screen-printed electrodes modified with DNA[J]. Analytica Chimica Acta, 2003, 479(2): 125–134.
Liu P G, Gong K C. Synthesis and characterization of polyaniline intercalated graphite oxide composite[J]. Acta Polymerica Sinica, 2000, 4: 492–495.
Beck F, Abdelmula E, Dahlhaus M. Anodic deposition of composites with a matrix of intrinsically conducting polymers[J]. Electrochimica Acta, 2000, 45(20): 3423–3429.
Del R, Zagal J H, Andrade G T, et al. Synthesis and characterization of a composite of polyaniline and carbon black[J]. Journal of Applied Electrochemistry, 1999, 29(6): 759–764.
Nunziante P, Pistoia G. Factors affecting the growth of thick polyaniline films by the cyclic voltammetry technique[J]. Electrochimica Acta, 1989, 34(2): 223–228.
Zotti G, Cattarin S, Comisso N. Cyclic potential sweep electropolymerization of aniline: The role of anions in the polymerization mechanism[J]. Journal of Electroanalytical Chemistry, 1988, 239(1/2): 387–396.
Shu F R, Wilson G S. Rotating ring-disk enzyme electrode for surface catalysis studies [J]. Analytical Chemistry, 1976, 48(12): 1679–1686.
Mu S L, Xue H G. Bioelectrochemical responses of the polyaniline glucose oxidase electrode[J]. Journal of Electroanalytical Chemistry, 1991, 304(1/2): 7–16.
Lu S Y, Li C F, Zhang D D, et al. Electron transfer on an electrode of glucose oxidase immobilized in polyaniline[J]. Journal of Electroanalytical Chemistry, 1994, 364(1/2): 31–36.
Yokoyama K, Tamiya E, Karube I. Kinetics of an amperometric glucose sensor with a soluble mediator[J]. Journal of Electroanalytical Chemistry, 1989, 273(1/2): 107–117.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Projects(50473022, 20673036) supported by the National Natural Science Foundation of China; project(2005) supported by the State Key Laboratory of Chemo/Biosensing and Chemometrics of China; project(2006FJ4100) supported by the Science Technology Project of Human Province; project(2006) supported by the Postdoctor Foundation of Hunan University
Rights and permissions
About this article
Cite this article
Zhou, Hh., Chen, H., Chen, Jh. et al. Polyaniline-graphite composite film glucose oxidase electrode. J Cent. South Univ. Technol. 13, 653–657 (2006). https://doi.org/10.1007/s11771-006-0010-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-006-0010-z