Abstract
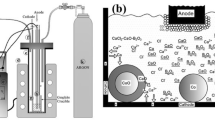
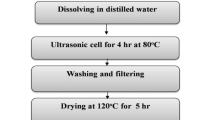
Spherical cobalt carbonate with high tap density, good crystallization and uniform particle size was prepared by controlled chemical crystal method using cobalt chloride and ammonium bicarbonate as cobalt source and precipitator. The effects of pH value and reaction time on crystallization and physical properties of cobalt carbonate were studied. The results show that the key factors influencing the preparation process of spherical cobalt carbonate with high tap density and good crystallization are how to control pH value (7.25 ± 0.05) and keep some reaction time (about 10 h). Co4O3 was prepared by sintering spherical morphology CoCO3 samples at varied temperatures. The results show that as the decomposition temperature increases, the as-obtained Co4O3 products with porous structure transform into polyhedral structure with glazed surface, and simultaneously the cobalt content and tap density increase. However, the specific surface area shows a trend of decrease.
Similar content being viewed by others
References
Furlanetto G. Precipation of spherical Co3O4 particals[J]. Journal of Colloid and Interface Science, 1995, 170: 169–175.
Baydi M E, Poillerat G, Rehspringer J L, et al. A Sol-Gel route for the preparation of Co3O4 catalyst for oxygen electrocatalysis in alkaline medium[J]. Journal of Solid State Chemistry, 1994, 109: 281–288.
YANG Yi-yong, SUN Ju-tang, YUAN Liang-jie, et al. Micro-Method powder X-ray diffraction analysis of thermal decomposition product of basic cobalt carbonate[J]. Journal of Wuhan University: Natural Science Edition, 2001, 47(6): 660–662. (in Chinese)
ZHONG Wen-bin, YANG Yu-xi, ZHANG Zhao. A study on the production of tricobalt tetroxide by wet chemistry thermodynamic analysis[J]. Sichuan Nonferrous Metals, 2000(1): 23–26.(in Chinese)
LIU Cheng. Preparation of cobalt oxide for lithium battery[J]. Nonferrous Metals, 2002, 54(4): 25–29. (in Chinese)
LI Ya-dong, HE Yun-pu, LI Long-quan, et al. Fabrication of Co3O4 ultrafines by a liquid-control-precipitation method[J]. Chemical Journal of Chinese University, 1999, 20(4): 519–522. (in Chinese)
Matijevic E. Production of mono-dispersed colloidal particles[J]. Ann Rev Mater Sci, 1985(15): 483.
Evic E M. Mono-dispersed inorganic colloids: achievements and problems[J]. Pure & Appl Chem, 1992, 64(11): 1703.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, J., Wang, Jf., Liu, Yd. et al. Preparation of spherical cobalt carbonate powder with high tap density. J Cent. South Univ. Technol. 13, 642–646 (2006). https://doi.org/10.1007/s11771-006-0008-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-006-0008-6