Abstract
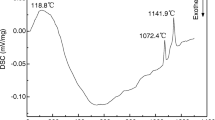
4.25Cu-0.75Ni/NiFe2O4 cermets were prepared by doping NiFe2O4 ceramic matrix with the mixed powders of Cu and Ni or Cu-Ni alloy powder as the electrical conducting metallic elements. The effects of technological parameters, such as the adding modes of metallic elements, the ball milling time, the sintering time and the sintering temperature, on the relative density and resistivity of the cermets were studied. The results show that the resistivity of 4.25Cu-0.75Ni/NiFe2O4 cermets decreases with increasing temperature, and has a turning point at 590 °C, which is similar to that of NiFe2O4 ceramic. The sintering temperature and adding modes of metallic elements have a great influence on the properties of 4.25Cu-0.75Ni/NiFe2O4 cermets. When the sintering temperature increases from 1200 °C to 1300 °C, the relative density increases from 89.86% to 95.33%, and the resistivity at 960 °C decreases from 0.11 Ω · cm to 0.03 Ω · cm, respectively. When the metallic elements are added with the mixed powders of Cu and Ni, the cermets of finely and uniformly dispersed metallic phase, high density and electric conductivity are obtained. The relative density and resistivity at 960 °C are 90.23% and 0.04 Ω · cm respectively for the cermet samples sintered at 1200 °C for 2 h, which are both better than those of the cermets prepared under the same technique conditions but with the metallic elements added as 85Cu-15Ni alloy powders.
Similar content being viewed by others
References
LIU Ye-xiang. Advance on the research and development of inert anode and wettable cathode in the aluminum electrolysis[J]. Light Metals, 2001, (5): 26–29. (in Chinese)
Pawlek R P. Inert anodes: an update[A]. Schneider W. Light Metals [C]. Warrendale, Pa: TMS, 2002. 449–456.
Gray P T. Corrosion and passivation of cermet inert anodes in cryolite-type electrolytes[A]. Miller R E. Light Metals[C]. Warrendale, Pa: TMS, 1986. 309–320.
Sekhar J A, Liu J, Deng H, et al. Graded nonconsumable anode materials[A]. Welch B. Light Meals[C]. Warreudale, Pa: TMS, 1998. 597–603.
YANG Bao-gang, YU Pei-zhi, YU Xian-jin, et al. Inert electrode materials for aluminum electrolysis[J]. Light Metals, 2000, (5): 32–35. (in Chinese)
Gregg J, Frederick M, King H. Testing of cerium oxide coated cermet anodes in a laboratory cell[A]. Mason D A. Light Metals[C]. Warreudale, Pa: TMS, 1993. 455–464.
QIN Qing-wei, LAI Yan-qing, ZHANG Gang, et al. Solid state reaction synthesis of Ni(1−x)Zn x Fe2O4 spinel used as matrix of inert anodes in aluminum electrolysis [J]. The Chinese Journal of Nonferrous Metals, 2003, 13 (3): 769–773. (in Chinese)
GUANG Zheng-duo. Physical Capability of Inorganic Materials[M]. Beijing: Tsinghua University Press, 2002.
WANG Ling-sen. Special Ceramics[M]. Changsha: Central South University of Technology Press, 1998. (in Chinese)
Elhiti M A, Aboelata A M. Semiconductivity in Ba2NI2−x Zn x Fe12O22 Y-type hexaferrites[J]. Journal of Magnetism and Magnetic Materials, 1999, 195 (3): 667–678.
WANG Chang-fu, LI Guo-xun. Influence of metal additives on the electrical conductivities of the oxide ceramics as an electrode material[J]. Rare Metals, 1993, 12(2): 126–130.
WANG Chang-fu, LI Guo-xun, QU Shu-ling, et al. Effect of adding Y2O3 in cermet electrodes on the electrical conductivities[J]. Rare Metals, 1992, 11 (4): 255–259.
XIONG Wei-hao, ZHOU Feng-yun, LI Guo-an, et al. The influence of powder particle size on the structure and properties of Ti(C, N)-based cermet [J]. The Journal of Huazhong University of Science and Technology (Natural Science), 1995, 23 (12): 37–42. (in Chinese)
HANG Pei-yun. Powder Metallurgical Theory [M]. Beijing: Metallurgical Industrial Press, 1997. (in Chinese)
TANG Ren-zheng. Physical Metallurgical Theory [M]. Beijing: Metallurgical Industrial Press, 1995. (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project (G1999064903) supported by the National Key Fundamental Research and Development Program of China; project(2001AA335013) supported by the National High Technology Research and Development Program of China; project (50204014) supported by the National Natural Science Foundation of China
Rights and permissions
About this article
Cite this article
Li, J., Zhang, G., Lai, Yq. et al. Preparation and properties of 4.25Cu-0.75Ni/NiFe2O4 cermet. J Cent. South Univ. Technol. 12, 284–289 (2005). https://doi.org/10.1007/s11771-005-0146-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11771-005-0146-2