Abstract
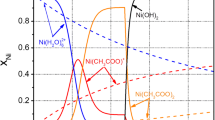
Electrochemical behaviors of Zn-Fe alloy and Zn-Fe-TiO2 composite electrodeposition in alkaline zincate solutions were studied respectively by the methods of linear potential sweep and cyclic voltammetry. From the results it can be concluded that Zn shows under potential deposition, Zn-Fe alloy codeposition is anomalous codeposition and Zn-Fe alloy cathode polarization is increased with the introduction of additive. From the view point of electrochemistry, the reasons that the content of Fe in the Zn-Fe coating changes with the composition of the electrolyte and the process conditions altering and the relationship between the content of Fe and the appearance of the coating are interpreted. The cathode polarization of Zn-Fe alloy codeposition is enhanced obviously with addition of additive. In the course of composite electrodeposition, TiO2 has less promotion to electrodeposition of zinc ions than to iron ions, while the electrodeposition of iron ions improves the content of TiO2 in composite coating, which is in agreement with the results of process experiments.
Similar content being viewed by others
References
GONG Zhu-qing. Introduction to Theory Electrochemistry(in Chinese)[M]. Changsha: Central South University of Technology Press, 1997. 180–182,139.
SHU Yu-de, CHEN Bai-zhen. Research Technique of Metallurgical Electrochemistry(in Chinese)[M]. Changsha: Central South University of Technology Press, 1990. 159–162, 87–94, 214–216, 277.
TIAN Zhao-wu. Research Methods of Electrochemistry (in Chinese) [M]. Beijing: Science Press, 1984. 240–246, 327.
WANG Yun-yan, SHU Yu-de, PENG Wen-jie, et al. Study on additive of Zn-Fe alloy electrodeposition in alkaline zincate solution[J]. Electroplating and Finishing (in Chinese), 2002,21(5):11–16.
WANG Yun-yan, PENG Wen-jie, CHAI Li-yuan, et al. Study on the process of Zn-Fe alloy electrodeposition in alkaline zincate solution[J]. Electroplating and Pollution(in Chinese), 2003,23(2):11–14.
WANG Yun-yan, PENG Wen-jie, HE De-wen, et al. Study on the process of Zn-Fe-TiO2 alloy electrodeposition in alkaline zincate solution[J]. Electroplating and Finishing(in Chinese), 2003, 22(1):19–23.
Mao Z, Srinivasan S, Appleby A J. Effect of lead monoxide on zinc electrodeposition from zincate solutions [J]. J Appl Electrochem Soc (in Chinese), 1992, 22(8):693–698.
ZHA Quan-xing. Introduction to Dynamics of Electrode Process(in Chinese)[M]. Beijing: Science Press, 1987. 407–415.
Barder A J, Fukna L R. Principle and Application of Electrochemistry Technique(in Chinese)[M]. Beijing: Chemical Industry Press, 1986.
HUANG Zi-qing. Theory Introduction to Electrolyte Solution(in Chinese) [M]. Beijing: Science Press, 1983. 84–86.
TU Zhen-mi. Principle and Techniques of Alloy Electroplating(in Chinese)[M]. Beijing: National Defense Industry Press, 1993.231–243.
DENG Zhao-yang. Study on Process and Foundational Theory of Zn-Co, Zn-Co-TiO2 Electrodeposition (in Chinese) [D]. Changsha: Central South University, 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Yy., Peng, Wj., Chai, Ly. et al. Electrochemical behaviors of Zn-Fe alloy and Zn-Fe-TiO2 composite electrodeposition. J Cent. South Univ. Technol. 10, 183–189 (2003). https://doi.org/10.1007/s11771-003-0005-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11771-003-0005-y