Abstract
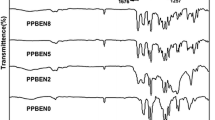
The effects of adding cosolvents of diglyme and 15-crown-5 to the reaction mixture of Wurtz-type coupling of dichlorosilanes on the yield and relative molecular mass dispersity of polymethylphenethylsilane(PMPES) were discussed. The results show that addition of 10% (volume ratio of diglyme to toluene) diglyme as a cosolvent to the reaction mixture leads to the yield increase of PMPES with a monomodal distribution of relative molecular masses. Adding 10% diglyme to the reaction mixtures, the yields of polymethylcyclohexylsilane(PMCS) and copolymers (polymethylphenethylsilane-co-methylcyclohexylsilane), (the molar ratios of methylphenethyldichlorosilane to methylcyclohexyldichlorosilane were 2.0, 1.0 and 0.5, and the copolymers were abbreviated by Copolymers I, II, III, respectively) are 47%, 52%, 54%, 53%, respectively. Their relative molecular masses \((\bar M_w )\)almost reach 105. These polysilanes were characterized by 1H-NMR, IR and UV absorption spectrum.
Similar content being viewed by others
References
West R. The polysilane high polymer[J]. J Organometallic Chemistry, 1986, 300: 327–346
Hiroshi B, Ken S. The importance of organosilane polymer photo-oxidation in resist pattern fabrication[J]. J Appl Polym Sci, 1987, 33: 2787–2793
Trefonas P, West R. Organosilane high polymer: synthesis of formable homopolymers[J]. J Polym Sci(Polym Lett Ed), 1983, 21: 819–822
Miller R D. Block interrupt polysilane derivatives[J]. J Polym Sci(Part A: Polym Chem), 1990, 28: 2665–2677
Miller R D, Michl J. Polysilane high polymers[J]. Chem Rev, 1989, 89: 1359–1410
Devaux J, Sledz J. Some observations about polysilane synthesis[J]. Eur Polym J, 1989, 25(3): 263–266
Gauthier S, Worsfold D J. The effect of phase-transfer catalysts on polysilane formation[J]. Macromolecules, 1989, 22: 2213–2218
Patricia P C Sartoratto, Valeria I, Yoshida P. Poly (dimethylsilyleneco-diphenyl-silylene)[J]. J Polym Sci(Part A: Polym Chem), 1992, 30(11): 2333–2340
HU Hui-ping, CHEN De-ben. Syntheses of methylphenethyldichlorosilane and methylcyclohexyldichlorosilane[J]. Chinese Chemical World, 1993, 34(7): 336
ZHANG X H, West R. Organosilane polymers[J]. J Polym Sci (Polym Chem Ed), 1984, 22: 225–238
ZHANG X H, West R. Formable copolymers containing diphenylsilylene units[J]. J Polym Sci(Polym Chem Ed), 1984, 22: 479–485
Author information
Authors and Affiliations
Additional information
Foundation item: The National Natural Science Foundation of China (No. 28970817)
Biography of the first author: HU Hui-ping, doctoral student, born in 1969, majoring in applied chemistry.
Rights and permissions
About this article
Cite this article
Hu, Hp., Huang, Kl., Pan, Cy. et al. Study on syntheses and characterizations of polymethylphenethylsilane, polymethylcyclohexylsilane and their copolymers. J Cent. South Univ. Technol. 7, 92–96 (2000). https://doi.org/10.1007/s11771-000-0040-x
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11771-000-0040-x