Abstract
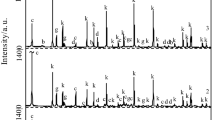
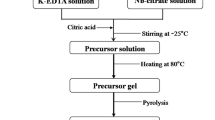
Kaolinite was hydrothermally synthesized from alumina gel and silicate by dissolving alumina gel in oxalic acid before it was mixed with silicate, effects of the amount of addition on the species of synthetic products were discussed. The reaction product was characterized by X-ray diffraction (XRD) and transmission electron microscopy (TEM). The results show that analcite is the only phase of the reaction solution without oxalic acid, the proportion of kaolinite in product increases with the amount of addition, and kaolinite is the main species when the molar ratio of oxalic acid to alumina reaches 0.6:1.0. This is because oxalic acid addition is beneficial to the formation of kaolinite through changing the coordination number of aluminium from four to six, while the mixture of alumina gel, before it was dissolved in oxalic acid with silicate interfered with the crystallization of kaolinite.
Similar content being viewed by others
References
Eberl D, Hower J. Kaolinite synthesis: The role of the Si/Al and (Alkali)/(H+) ratio in hydrothermal systems[J]. Clays & Clay Minerals, 1975, 23(4): 301–309
De Vynck Y A. Action des ions alcalins sur la transformation hudrothermal de gels silico-alumineux, Premiére partie: Influence de l’ion Li+ [J]. Silicates Industriels (in French), 1975, 40: 259–272
De Vynck Y A. Action des ions alcalins sur la transformation hudrothermal de gels silico-alumineux, Premiére partie: Influence de l’ion K+[J]. Silicates Industriels (in French), 1976, 41: 67–81
Miyawaki R, Tomura S, Shibasaki Y, et al. Appropriate pH for hydrothermal synthesis of kaolinite from amorphous mixture of alumina and silica[J]. Reports of Government Industrial Research Institute, Nagoya Kogyo Gijutsu Shikensho Hokoku (in Japanese), 1989, 38(12): 330–335
Miyawaki R, Ota K, Tanaka K, et al. Factor of acidification of reaction system during hydrothermal synthesis of kaolinite from amorphous mixture of alumina and silica[J]. Reports of Government Industrial Research Institute, Nagoya Kogyo Gijutsu Shikensho Hokoku (in Japanese), 1990, 39(14):490–497
Shibasaki. Effect of acidity on the hydrothermal synthesis of kaolinite from silica-gel and gibbsite[J]. Clays & Clay Minerals, 1996, 44(5):417–423
Miyawaki R, Tomura S, Shibasaki Y, et al. Effects of solution chemistry on the hydrothermal synthesis of kaolinite[J]. Clays & Clay Minerals, 1991, 39(5):498–508
Author information
Authors and Affiliations
Additional information
Foundation item: The Natural Science Foundation of Hunan Province (No. 01-941)
Biography of the first author: LIU Su-qin, born in May 1966, majoring in functional material chemistry.
Rights and permissions
About this article
Cite this article
Liu, Sq., Huang, Kl. Effects of oxalic acid addition on the hydrothermal synthesis of kaolinite. J Cent. South Univ. Technol. 7, 34–36 (2000). https://doi.org/10.1007/s11771-000-0009-9
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11771-000-0009-9